Efficacy and Safety of Once Daily Dosing vs. Approved On/Off Dosing of Edaravone Oral Suspension Up to 48 Weeks in Patients With Amyotrophic Lateral Sclerosis (Study MT-1186-A02)
- PMID: 40474686
- PMCID: PMC12338024
- DOI: 10.1002/mus.28448
Efficacy and Safety of Once Daily Dosing vs. Approved On/Off Dosing of Edaravone Oral Suspension Up to 48 Weeks in Patients With Amyotrophic Lateral Sclerosis (Study MT-1186-A02)
Abstract
Introduction/aims: An On/Off dosing regimen of intravenous (IV) edaravone and edaravone oral suspension is approved in the US for the treatment of amyotrophic lateral sclerosis (ALS). Placebo-controlled clinical trials showed IV edaravone slows the rate of physical functional decline. This study evaluated whether investigational daily dosing displayed superior efficacy vs. approved on/off dosing of edaravone oral suspension, and assessed safety and tolerability, over 48 weeks in patients with ALS.
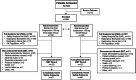
Methods: Study MT-1186-A02 (NCT04569084) was a multicenter, phase 3b, double-blind, parallel group, superiority study that randomized patients to edaravone oral suspension (105-mg dose) administered Once Daily or the same edaravone oral suspension dose administered according to the approved On/Off regimen including placebo to mimic daily drug dosing. Patients had definite or probable ALS, baseline forced vital capacity ≥ 70%, and baseline disease duration ≤ 2 years. The primary endpoint was a combined assessment of function and survival (CAFS) at week 48, which included change in ALS Functional Rating Scale-Revised (ALSFRS-R) and time to death.
Results: CAFS at week 48 indicated Once Daily dosing did not show a statistically significant difference vs. approved on/off dosing (p = 0.777). Both dosing regimens provided comparable change from baseline ALSFRS-R total score to week 48 (least squares mean difference: 0.27 [95% CI -1.43 to 1.97]). Edaravone oral suspension was well tolerated, and no new safety concerns were identified in either group.
Discussion: Daily edaravone oral suspension did not show superiority and had equivalent safety and tolerability vs. the approved On/Off regimen, reinforcing the appropriateness of the approved dosing regimen.
Keywords: amyotrophic lateral sclerosis; clinical trial; edaravone; efficacy; safety.
© 2025 The Author(s). Muscle & Nerve published by Wiley Periodicals LLC.
Conflict of interest statement
J.R. is a consultant for Expansion Therapeutics, National Institutes of Health, Department of Defense, F Prime, The ALS Association; A.G. has served as a consultant for Mitsubishi Tanabe Pharma Inc.; S.D. and M.C. have nothing to disclose; L.Z. has received honoraria for consulting with MTP, Biogen, Amylyx, and Cytokinetics; A.C. serves on scientific advisory boards for Mitsubishi Tanabe, Roche, Biogen, Denali Pharma, A.C. Immune, Biogen, Lilly, and Cytokinetics and has received a research grant from Biogen; G.S., H.Y., and M.A. have served as medical advisors for Mitsubishi Tanabe Pharma Corporation; M.D. is a medical advisor for the MT‐1186‐A02 study; V.T. is an employee of Mitsubishi Tanabe Pharma Europe Ltd.; N.S. has served as a statistical consultant for NeuroDerm and Mitsubishi Tanabe Pharma Inc.; D.S., F.T., A.S., A.W., M.H., and S.A. are employees of Mitsubishi Tanabe Pharma America Inc.
Figures


References
-
- Rilutek (Riluzole). Prescribing Information (Covis Pharmaceuticals, Inc., 2016).
-
- Radicava (Edaravone) IV and Radicava ORS (Edaravone) Oral Suspension. Prescribing Information (Mitsubishi Tanabe Pharma Corporation, 2022).
-
- QALSODY (Tofersen) Injection. Prescribing Information (Biogen MA Inc., 2023).
-
- Writing Group on behalf of the Edaravone ALS Study 19 Group , “Safety and Efficacy of Edaravone in Well Defined Patients With Amyotrophic Lateral Sclerosis: A Randomised, Double‐Blind, Placebo‐Controlled Trial,” Lancet Neurology 16, no. 7 (2017): 505–512. - PubMed
-
- Cedarbaum J. M., Stambler N., Malta E., et al., “The ALSFRS‐R: A Revised ALS Functional Rating Scale That Incorporates Assessments of Respiratory Function. BDNF ALS Study Group (Phase III),” Journal of the Neurological Sciences 169, no. 1–2 (1999): 13–21. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

