Biopsy in chronic liver disease: proposal for a shared path between clinicians and pathologists
- PMID: 40474712
- PMCID: PMC12142296
- DOI: 10.32074/1591-951X-1063
Biopsy in chronic liver disease: proposal for a shared path between clinicians and pathologists
Abstract
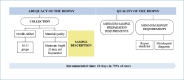
Introduction: Liver biopsy is fundamental for characterizing chronic liver disease. Effective communication between specialists during the diagnostic process is crucial. This project aims to outline a diagnostic path shared by clinicians and pathologists, and to propose practical solutions at different stages of the diagnostic work-up, from clinical suspicion to the histology report in patients with chronic liver diseases.
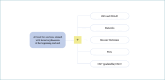
Methods: A panel of experts, within the methodological framework of lean management, joined two rounds of discussion sharing their professional experiences. They reached an agreement on the essential phases and actions of the diagnostic process, and built a shared diagnostic workflow.
Results: The panel agreed on the importance of a standardized form to be filled with all relevant clinical and laboratory data to ensure the flow of information between the clinician and the pathologist. Further decisions were reached on the following practical issues: the advantage of performing liver biopsies in dedicated centers, the need for homogeneous procedures, and the minimum quality standards in all phases, including reporting. Finally, the panel agreed on the usefulness of digital pathology to exchange observations and opinions and to create a territorial network to discuss challenging cases.
Conclusion: Sharing a diagnostic path between the pathologist and the clinician can be a powerful tool to improve both the timing and accuracy of the histology report.
Keywords: Chronic liver diseases; diagnostic pathway; lean management; liver biopsy.
Copyright © 2025 Società Italiana di Anatomia Patologica e Citopatologia Diagnostica, Divisione Italiana della International Academy of Pathology.
Conflict of interest statement
The authors declare no conflict of interest
Figures

Similar articles
-
Second opinion pathology in liver biopsy interpretation.Am J Gastroenterol. 2001 Nov;96(11):3158-64. doi: 10.1111/j.1572-0241.2001.05273.x. Am J Gastroenterol. 2001. PMID: 11721765
-
Audit of medical (non-targeted) liver biopsy specimen quality, pathology reporting and effect on patient management.J Clin Pathol. 2022 Jul;75(7):498-502. doi: 10.1136/jclinpath-2020-207366. Epub 2021 May 26. J Clin Pathol. 2022. PMID: 34039666
-
Utility of AI digital pathology as an aid for pathologists scoring fibrosis in MASH.J Hepatol. 2025 May;82(5):898-908. doi: 10.1016/j.jhep.2024.11.032. Epub 2024 Nov 28. J Hepatol. 2025. PMID: 39612947 Clinical Trial.
-
Mistakes in utilising histopathology for the management of liver disease.Expert Rev Gastroenterol Hepatol. 2024 Apr-May;18(4-5):147-153. doi: 10.1080/17474124.2024.2355168. Epub 2024 May 15. Expert Rev Gastroenterol Hepatol. 2024. PMID: 38743469 Review.
-
[Value of liver histology in diagnosis of chronic hepatopathies].Praxis (Bern 1994). 1998 Oct 14;87(42):1397-401. Praxis (Bern 1994). 1998. PMID: 9824946 Review. German.
References
-
- Rockey DC, Caldwell SH, Goodman ZD, et al. American Association for the Study of Liver Diseases . Liver biopsy. Hepatology. 2009. Mar;49(3):1017-44. https://doi.org/10.1002/hep.22742. PMID: 19243014. 10.1002/hep.22742 - DOI - PubMed
-
- Boyd A, Cain O, Chauhan A, Webb GJ. Medical liver biopsy: background, indications, procedure and histopathology. Frontline Gastroenterol. 2020. Jan;11(1):40-47. https://doi.org/10.1136/flgastro-2018-101139. Epub 2019 Mar 2. PMID: 31885839; PMCID: PMC6914302. 10.1136/flgastro-2018-101139 - DOI - PMC - PubMed
-
- European Association for the Study of the Liver. Electronic address: easloffice@easloffice.eu; Clinical Practice Guideline Panel; Chair; EASL Governing Board representative; Panel members: EASL Clinical Practice Guidelines on non-invasive tests for evaluation of liver disease severity and prognosis - 2021 update. J Hepatol. 2021. Sep;75(3):659-689. https://doi.org/10.1016/j.jhep.2021.05.025. Epub 2021 Jun 21. PMID: 34166721. 10.1016/j.jhep.2021.05.025 - DOI - PubMed
-
- Goodman ZD. Grading and staging systems for inflammation and fibrosis in chronic liver diseases. J Hepatol. 2007. Oct;47(4):598-607. https://doi.org/10.1016/j.jhep.2007.07.006. Epub 2007 Jul 30. PMID: 17692984. 10.1016/j.jhep.2007.07.006 - DOI - PubMed
-
- Cadranel JF, Nousbaum JB. Indications de la ponction biopsie hépatique au cours des maladies parenchymateuses diffuses du foie [Current trends in liver biopsy indications in chronic liver diseases]. Presse Med. 2012. Nov;41(11):1064-70. French. https://doi.org/10.1016/j.lpm.2012.01.034. Epub 2012 Mar 15. PMID: 22425478. 10.1016/j.lpm.2012.01.034 - DOI - PubMed
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

