Expert perspectives on the use of safinamide for Parkinson's disease in Portugal: insights from a Portuguese Delphi Consensus
- PMID: 40474741
- PMCID: PMC12224116
- DOI: 10.57264/cer-2024-0228
Expert perspectives on the use of safinamide for Parkinson's disease in Portugal: insights from a Portuguese Delphi Consensus
Abstract
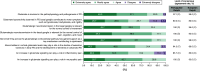
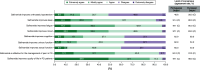
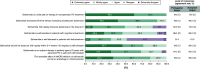
Aim: Safinamide is an approved medication for managing motor fluctuations in Parkinson's disease (PD). However, limited data exist regarding its application in clinical practice in Portugal and the perspectives of Portuguese neurologists on its use. To address this, a group of Portuguese specialists with recognized expertise in PD management convened to compile the insights on various aspects of safinamide use in PD patients among the field and to develop recommendations aimed at informing and guiding physicians in Portugal on its optimal clinical application. Materials & methods: A focus group composed of nine Portuguese PD experts developed a questionnaire building on the 2022 European Delphi study, and employed a Delphi methodology approach to gather the views of Portuguese neurologists with a minimum of 5 years of clinical experience with safinamide (n = 35). A final online questionnaire comprising 35 statements was administered in a single-round Delphi format, utilizing a 5-point Likert scale. Consensus was defined as achieving ≥66% agreement or disagreement among the panelists. Results: A strong consensus emerged among Portuguese neurologists regarding the therapeutic efficacy of safinamide in addressing motor symptoms, motor fluctuations and quality of life. Additionally, agreement was reached on its positive effects on nonmotor symptoms such as sleep, fatigue, mood, quality of life and pain management. However, no consensus was achieved regarding safinamide's efficacy in managing orthostatic hypotension, cognitive issues, urinary and sexual dysfunction, as well as its safety profile in PD patients with hallucinations. Overall, the opinions of Portuguese neurologists aligned closely with those of their European counterparts. Conclusion: This Delphi study highlights the consensus among Portuguese neurologists on the efficacy of safinamide for managing motor symptoms in PD. The considerations presented herein offer essential guidance for effectively managing PD with safinamide, ultimately enhancing patient care in Portugal.
Keywords: Delphi; Parkinson’s disease; Portugal; dyskinesia; efficacy; fluctuations; motor symptoms; nonmotor symptoms; safety; safinamide.
Conflict of interest statement
Competing interests disclosure
AM Rodrigues received honoraria from AbbVie, BIAL – Portela & Ca, S.A., Italfarmaco and Zambon S.A.U. C Costa received honoraria from Allergan, BIAL – Portela & Ca, S.A. and Zambon S.A.U. M Gago received honoraria from AbbVie, BIAL – Portela & Ca, S.A. and Zambon S.A.U. M Grunho received honoraria from AbbVie, BIAL – Portela & Ca, S.A., Biogen, Johnson & Johnson Innovative Medicine – Janssen, Merck, Novartis, Sanofi and Zambon S.A.U. LC Guedes received honoraria from AbbVie, BIAL – Portela & Ca, S.A., Roche and Zambon S.A.U. A Morgadinho received honoraria from AbbVie, BIAL – Portela & Ca, S.A. and Zambon, S.A.U. MJ Rosas received honoraria from Zambon, S.A.U. R Simões received honoraria from AbbVie, BIAL – Portela & Ca, S.A. and Zambon, S.A.U. AG Velon does not have any conflicts of interest to declare. The authors have no other competing interests or relevant affiliations with any organization or entity with the subject matter or materials discussed in the manuscript apart from those disclosed.
The authors have no other competing interests or relevant affiliations with any organization or entity with the subject matter or materials discussed in the manuscript apart from those disclosed.
Figures




References
-
- Saini N, Singh N, Kaur N et al. Motor and non-motor symptoms, drugs, and their mode of action in Parkinson's disease (PD): a review. Med. Chem. Res. 33(4), 580–599 (2024).
-
- van der Meer F, Jorgensen J, Hiligsmann M. Burden of non-motor symptoms of Parkinson's disease: cost-of-illness and quality-of-life estimates through a scoping review. Expert Rev. Pharmacoecon. Outcomes Res. 25(1), 17–27 (2025). - PubMed
-
- Ahlskog JE. Parkinson's disease progression is multifaceted: evidence for the underlying benchmarks. Parkinsonism Relat. Disord. 121, 106037 (2024). - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
