Investigation of HO-1 Regulation of Liver Fibrosis Related to Nonalcoholic Fatty Liver Disease Through the SIRT1/TGF-ß/Smad3 Pathway
- PMID: 40474887
- PMCID: PMC12134905
- DOI: 10.14218/JCTH.2024.00481
Investigation of HO-1 Regulation of Liver Fibrosis Related to Nonalcoholic Fatty Liver Disease Through the SIRT1/TGF-ß/Smad3 Pathway
Abstract
Background and aims: Heme oxygenase 1 (HO-1) has an influential yet insufficiently investigated effect on Sirtuin 1 (SIRT1), a histone deacetylase activated by nicotinamide adenine dinucleotide, which may impact the transforming growth factor-β (TGF-ß)/Smad3 pathway in nonalcoholic fatty liver disease (NAFLD)-related liver fibrosis. This study aimed to elucidate the regulation of NAFLD-related liver fibrosis induced by HO-1 through the SIRT1/TGF-ß/Smad3 pathway.
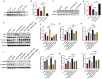
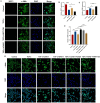
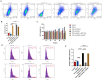
Methods: HO-1 induction and inhibition were established in C57BL/6J mice fed a methionine- and choline-deficient (MCD) diet. Additionally, wild-type mice were fed either a normal diet or an MCD diet. Hematoxylin and eosin, Masson's trichrome, and Sirius Red staining were used to assess hepatic steatosis, inflammation, and fibrosis. In vitro, plasmid overexpression and small interfering RNA silencing of HO-1 were performed in LX-2 cells. Cell viability was assessed using the Cell Counting Kit-8, and apoptosis was evaluated via terminal deoxynucleotidyl transferase dUTP nick-end labeling and immunofluorescence. Flow cytometry was employed to assess apoptosis and reactive oxygen species production. Western blot and real-time quantitative reverse transcription polymerase chain reaction were used to analyze the mRNA and protein expression of genes related to HO-1, SIRT1, the TGF-ß signaling pathway, and fibrosis.
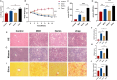
Results: MCD-fed mice developed significant liver damage, including steatosis, inflammatory infiltration, and pericellular fibrosis. Zinc protoporphyrin treatment exacerbated these conditions. Corroborating these findings, silencing HO-1 in LX-2 cells increased the expression of fibrosis-related genes. Furthermore, HO-1 overexpression not only increased SIRT1 expression but also reduced the activity of key regulatory factors in the TGF-ß signaling pathway, suggesting a potential interaction between HO-1 and the SIRT1/TGF-ß pathway.
Conclusions: HO-1 inhibits the activation of the TGF-ß/Smad3 pathway in NAFLD-related liver fibrosis through SIRT1. These findings provide insights into new therapeutic strategies for treating NAFLD-associated liver fibrosis.
Keywords: HO-1; Heme oxygenase-1; Non-alcoholic steatohepatitis-related liver fibrosis; SIRT1; Sirtuin 1; TGF-ß pathway.
© 2025 Authors.
Conflict of interest statement
YN has been an Editorial Board Member of Journal of Clinical and Translational Hepatology since 2022. The other authors have no conflict of interests related to this publication.
Figures


Similar articles
-
Hydrogen-rich water protects against liver injury in nonalcoholic steatohepatitis through HO-1 enhancement via IL-10 and Sirt 1 signaling.Am J Physiol Gastrointest Liver Physiol. 2021 Apr 1;320(4):G450-G463. doi: 10.1152/ajpgi.00158.2020. Epub 2021 Jan 13. Am J Physiol Gastrointest Liver Physiol. 2021. PMID: 33439102
-
MicroRNA-29a Disrupts DNMT3b to Ameliorate Diet-Induced Non-Alcoholic Steatohepatitis in Mice.Int J Mol Sci. 2019 Mar 26;20(6):1499. doi: 10.3390/ijms20061499. Int J Mol Sci. 2019. PMID: 30917489 Free PMC article.
-
Heme Oxygenase-1 Suppresses Wnt Signaling Pathway in Nonalcoholic Steatohepatitis-Related Liver Fibrosis.Biomed Res Int. 2020 May 1;2020:4910601. doi: 10.1155/2020/4910601. eCollection 2020. Biomed Res Int. 2020. PMID: 32461992 Free PMC article.
-
Evaluation of lung protection of Sanghuangporus sanghuang through TLR4/NF-κB/MAPK, keap1/Nrf2/HO-1, CaMKK/AMPK/Sirt1, and TGF-β/SMAD3 signaling pathways mediating apoptosis and autophagy.Biomed Pharmacother. 2023 Sep;165:115080. doi: 10.1016/j.biopha.2023.115080. Epub 2023 Jun 29. Biomed Pharmacother. 2023. PMID: 37392658
-
The ménage à trois of autophagy, lipid droplets and liver disease.Autophagy. 2022 Jan;18(1):50-72. doi: 10.1080/15548627.2021.1895658. Epub 2021 Apr 2. Autophagy. 2022. PMID: 33794741 Free PMC article. Review.
References
-
- Maines MD, Trakshel GM, Kutty RK. Characterization of two constitutive forms of rat liver microsomal heme oxygenase. Only one molecular species of the enzyme is inducible. J Biol Chem. 1986;261(1):411–419. - PubMed
LinkOut - more resources
Full Text Sources
