Efficacy and safety of Xiao'er Fengre Qing oral liquid versus Oseltamivir in treating pediatric influenza (wind-heat invading the defense syndrome): a multicenter, randomized, non-inferiority trial
- PMID: 40474974
- PMCID: PMC12137347
- DOI: 10.3389/fphar.2025.1584003
Efficacy and safety of Xiao'er Fengre Qing oral liquid versus Oseltamivir in treating pediatric influenza (wind-heat invading the defense syndrome): a multicenter, randomized, non-inferiority trial
Abstract
Background: Xiao'er Fengre Qing Oral Liquid (XFQOL) is developed based on the classical traditional Chinese medicinal formula Yinqiao Powder. Compared to the original formulation, XFQOL exhibits enhanced heat-clearing, detoxification, and fever reduction, which can effectively address the common complications associated with influenza in children and is well-suited for pediatric use. However, there is currently a lack of high-quality evidence from clinical trials to support its efficacy and safety in clinical applications.
Objective: This study aimed to investigate the efficacy and safety of XFQOL compared with Oseltamivir in pediatric influenza.
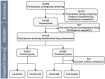
Methods: A multicenter, block-randomized, double-blind, double-dummy, positive-drug-controlled, non-Inferiority clinical trial design was conducted. The study plans to enroll 420 pediatric participants, with 210 in each group. The experimental group will receive XFQOL with an Oseltamivir granules placebo, and the control group will receive Oseltamivir granules with a XFQOL placebo for 5 days, followed by a 2-day post-treatment observation. The primary endpoint was clinical recovery time, while secondary endpoints included complete fever resolution time, the area under the curve (AUC) of Canadian Acute Respiratory Illness and Flu Scale (CARIFS) symptom dimension Score over time, Traditional Chinese Medicine (TCM) syndrome efficacy, disappearance rates for individual symptoms, incidences of complications and severe and critical influenza, the usage of acetaminophen, and viral negative conversion rate. Safety evaluation focused on adverse events (AE) and adverse drug reactions (ADR).
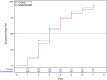
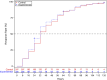
Results: A total of 418 participants were included in the Full Analysis Set, with 208 in the experimental group and 210 in the control group. Baseline characteristics were comparable between the groups. The median time to clinical recovery was 3 days for both groups, with a hazard ratio and its 95% confidence interval (experimental group/control group) of 1.115 (95% CI: 0.912-1.363). Non-inferiority testing demonstrated that the experimental group was not inferior to the control group. Subgroup analyses (positive for RT-PCR influenza, positive for RT-PCR influenza A, positive for RT-PCR influenza B) yielded results consistent with the primary endpoint. The median time to complete fever resolution was 32 h in both groups, with no statistically significant difference (P = 0.407). There were no statistically significant differences in the AUC of CARIFS symptom scores over time between the groups (P = 0.211). No significant differences were observed between the groups in the efficacy rates of TCM syndromes of Wind-Heat Invading the Defense Syndrome (P = 0.076) and Fright-complicated Syndrome (P = 0.168); however, significant differences were found in Phlegm-complicated Syndrome (P = 0.008) and Food-stagnation-complicated Syndrome (P = 0.024). The disappearance rates for individual symptoms, such as red and swollen pharynx, cough, copious sputum or audible phlegm sounds in the throat, and lack of appetite, showed statistically significant differences between the groups (P < 0.05), while no significant differences were observed for other symptoms. No statistically significant differences were observed between the experimental and control groups in the incidence of complications and severe and critical influenza, the usage of acetaminophen, and viral negative conversion rate (P > 0.05). The incidence rates of AE (P = 0.885) and ADR (P = 0.685) were comparable between the two groups, with no statistically significant differences observed.
Conclusion: The efficacy of XFQOL in treating pediatric influenza (Wind-Heat Invading the Defense Syndrome) is non-inferior to Oseltamivir with respect to clinical recovery time. Additionally, its effectiveness in terms of fever reduction, symptom alleviation, incidences of complications and severe and critical influenza, the usage of acetaminophen, and viral negative conversion rate is comparable to that of Oseltamivir. Furthermore, it demonstrates good safety, suggesting its potential for clinical application.
Clinical trial registration: clinicaltrials.gov, identifier ChiCTR2300076191.
Keywords: Xiao’er Fengre Qing oral Liquid; influenza; multicenter; pediatrics; randomized controlled trial; traditional Chinese medicine; wind-heat invading the defense Syndrome.
Copyright © 2025 Guo, Li, Zheng, Zhong, Xiong, Ming, Ding, Yan, Zhang, Zhou, Fu, Wang, Wang, Wang, Yang, Liu, Cai, Ning, Liu, Zhu, Gai, Liu, Sun, Wang, Li, Tian, Zhang, Guan, Li, Li, Liu, Kuang, Lu, Gao, Liang, Shen and Ma.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

References
-
- Centers for Disease Control and Prevention (2024). Clinical practice guidelines for influenza. Available online at: https://www.cdc.gov.
-
- Chen Y., Zhang T., Chen C., Wang X., Jiang Q. (2021). Research progress on chemical constituents,extraction technology and pharmacological effects of Saposhnikovia divaricate. Jiangsu Agric. Sci. 49 (09), 43–48. 10.15889/j.issn.1002-1302.2021.09.007 - DOI
-
- China Association of Chinese Medicine. Guideline for TCM pediatrics clinical diagnosis and treatment (2012). Beijing: China Traditional Chinese Medicine Press, 1–4.
LinkOut - more resources
Full Text Sources
Research Materials

