Progress in visible-light-activated photocatalytic coatings to combat implant-related infections: From mechanistic to translational roadmap
- PMID: 40475082
- PMCID: PMC12137198
- DOI: 10.1016/j.bioactmat.2025.04.037
Progress in visible-light-activated photocatalytic coatings to combat implant-related infections: From mechanistic to translational roadmap
Abstract
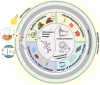
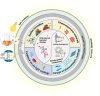
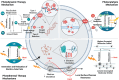
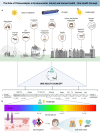
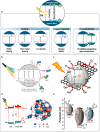
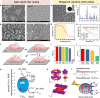
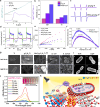
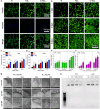
Biomedical and dental implants have enhanced healthcare but concurrently increased the risk of infections. Innovations in smart biomaterials, especially those responding to light stimuli through photocatalytic mechanisms, are emerging as promising solutions for activating targeted antimicrobial responses. While extensive reviews have provided insight into photocatalysis and its medical and environmental applications, limited focus has been given to solutions specifically tailored for implant contexts. The recent introduction of photocatalysis in the implant field, particularly visible-light-triggered photocatalytic coatings, represents a versatile approach to managing infections. These coatings offer on-demand reactive oxygen species generation, delivering antibacterial effects against a range of pathogens. Hence, this comprehensive review aims to summarize the latest advancements in design principles, physicochemical modifications, and surface optimizations, along with novel research concepts towards the achievement of visible-light-triggered photocatalytic antibacterial activity. Moreover, through a systematic search, this review discusses the current state-of-the-art regarding the antimicrobial efficacy of these biomaterials and the key factors influencing their performance, including microorganism type, photocatalyst properties, light source and intensity, and exposure time. Finally, it provides an in-depth discussion of current challenges, future directions, and regulatory considerations targeting biofilm-related implant treatments, offering guidance for future clinical adoption of multifunctional photocatalytic coatings in implant therapy.
Keywords: Biofilms; Coatings; Implants; Light; Photocatalysis.
© 2025 The Authors.
Conflict of interest statement
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures











References
-
- Magill S.S., Edwards J.R., Bamberg W., Beldavs Z.G., Dumyati G., Kainer M.A., Lynfield R., Maloney M., McAllister-Hollod L., Nadle J., Ray S.M., Thompson D.L., Wilson L.E., Fridkin S.K. Multistate point-prevalence survey of health care–associated infections. N. Engl. J. Med. 2014;370:1198–1208. doi: 10.1056/NEJMoa1306801. - DOI - PMC - PubMed
Publication types
LinkOut - more resources
Full Text Sources

