Dental follicle stem cell-derived small extracellular vesicles ameliorate pulpitis by reprogramming macrophage metabolism
- PMID: 40475085
- PMCID: PMC12137177
- DOI: 10.1016/j.bioactmat.2025.04.034
Dental follicle stem cell-derived small extracellular vesicles ameliorate pulpitis by reprogramming macrophage metabolism
Abstract
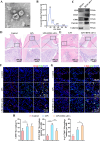
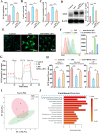
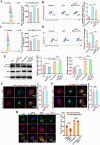
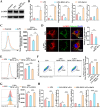
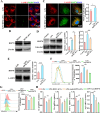
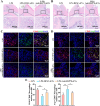
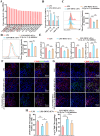
Vital pulp therapy (VPT) is considered a conservative means of preserving the vitality and function of the dental pulp after injury. However, current VPT has unfavorable effects on inflamed pulp. Mesenchymal stem cell (MSC)-derived small extracellular vesicles (MSC-sEVs) show powerful immunomodulatory capacities and exert therapeutic effects on a variety of inflammatory diseases. However, whether MSC-sEVs ameliorate the inflammatory response and promote inflammatory pulp repair in pulpitis is largely unknown. In this study, we show that sEVs derived from dental follicle stem cells (typical dental MSCs, DFSC-sEVs) alleviate lipopolysaccharide-induced pulpitis in rats and enhance pulp repair by inducing M2 macrophage polarization. Mechanistically, heat shock protein 70 (HSP70) within DFSC-sEVs can be supplemented into lysosomes to directly protect lysosomal function and induce mitophagy to promote the degradation of depolarized mitochondria, thereby preprogramming inflammatory macrophages to commit to oxidative phosphorylation, which fuels M2 polarization. Furthermore, DFSC-sEVs also transfer antioxidant miRNAs, including miR-24-3p and let-7c-5p, to inhibit mitochondrial reactive oxygen species production, thereby indirectly stabilizing lysosomes to induce M2 macrophage generation. Our study reveals a promising immunotherapeutic potential of DFSC-sEVs for VPT in inflamed pulp and a novel role for DFSC-sEVs in inhibiting the macrophage inflammatory response by protecting lysosomes and inducing mitophagy-mediated metabolic shifts toward oxidative phosphorylation.
Keywords: Dental follicle stem cell; Immunometabolism; Macrophage; Pulpitis; Small extracellular vesicle.
© 2025 The Authors.
Conflict of interest statement
He Liu is an editorial board member for Bioactive Materials and was not involved in the editorial review or the decision to publish this article. Ya Shen is an associate editor for Bioactive Materials and was not involved in the editorial review or the decision to publish this article. All authors declare that there are no competing interests.
Figures
References
-
- Duncan H.F., Galler K.M., Tomson P.L., Simon S., El-Karim I., Kundzina R., Krastl G., Dammaschke T., Fransson H., Markvart M., Zehnder M., Bjørndal L. European Society of Endodontology position statement: management of deep caries and the exposed pulp. Int. Endod. J. 2019;52:923–934. doi: 10.1111/iej.13080. - DOI - PubMed
-
- Kim D.-H., Jang J.-H., Lee B.-N., Chang H.-S., Hwang I.-N., Oh W.-M., Kim S.-H., Min K.-S., Koh J.-T., Hwang Y.-C. Anti-inflammatory and mineralization effects of ProRoot MTA and endocem MTA in studies of human and rat dental pulps in vitro and in vivo. J. Endod. 2018;44:1534–1541. doi: 10.1016/j.joen.2018.07.012. - DOI - PubMed
LinkOut - more resources
Full Text Sources

