Experience of a Nationwide implementation of single-tablet second-generation integrase inhibitors-based treatment in Mexico
- PMID: 40475094
- PMCID: PMC12138914
- DOI: 10.1016/j.ijregi.2025.100645
Experience of a Nationwide implementation of single-tablet second-generation integrase inhibitors-based treatment in Mexico
Abstract
Objectives: In Mexico, until 2018, antiretroviral therapy (ART) primarily relied on efavirenz-containing regimens or other options suitable for optimization, with ART costs among the highest in the region. This study evaluated the impact of a national strategy using single-tablet, second-generation integrase inhibitors (STSGII)-either bictegravir, emtricitabine, and tenofovir alafenamide or abacavir/lamivudine/dolutegravir-for ART initiation or switch.
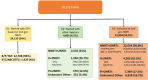
Methods: Adults initiating or switching to STSGII between June 1, 2019, and June 30, 2021, at clinics for individuals without social security, were categorized into three groups: G1 (initiated with STSGII), G2 (initiated with non-STSGII), and G3 (switched to STSGII). The switch was defined as individuals changing ART in the presence of virological suppression. Viral suppression (VS) at 6 months post-initiation or switch was reported. A Cox model evaluated VS and regimen durability, defined as maintaining VS without ART change, loss to follow-up, or death.
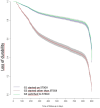
Results: A total of 70,732 individuals were included; 24,133 (34.1%) on G1, 4605 (6.5%) on G2, and 41,994 (59.4%) on G3. VS was achieved in 85.7%, 52.7%, and 82.7% of individuals in G1, G2, and G3, respectively. Durability was met in 85.1%, 41.3%, and 90.8% of individuals in G1, G2, and G3.
Conclusions: Second-generation INSTIs are effective and durable in this 2-year follow-up analysis, both for ART initiation and optimization in Mexico's population, offering a valuable strategy for improving ART outcomes.
Keywords: HIV; Mexico; Second-generation single-tablet integrase inhibitors.
© 2025 The Authors. Published by Elsevier Ltd on behalf of International Society for Infectious Diseases.
Conflict of interest statement
AP reports a relationship with Gilead Sciences that includes consulting or advisory and funding grants. AP reports a relationship with ViiV Healthcare that includes consulting or advisory and funding grants. All other authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures

Similar articles
-
Bictegravir, emtricitabine, and tenofovir alafenamide versus dolutegravir, abacavir, and lamivudine for initial treatment of HIV-1 infection (GS-US-380-1489): a double-blind, multicentre, phase 3, randomised controlled non-inferiority trial.Lancet. 2017 Nov 4;390(10107):2063-2072. doi: 10.1016/S0140-6736(17)32299-7. Epub 2017 Aug 31. Lancet. 2017. PMID: 28867497 Clinical Trial.
-
Bictegravir combined with emtricitabine and tenofovir alafenamide versus dolutegravir, abacavir, and lamivudine for initial treatment of HIV-1 infection: week 96 results from a randomised, double-blind, multicentre, phase 3, non-inferiority trial.Lancet HIV. 2019 Jun;6(6):e355-e363. doi: 10.1016/S2352-3018(19)30077-3. Epub 2019 May 5. Lancet HIV. 2019. PMID: 31068270 Clinical Trial.
-
Switching to fixed-dose bictegravir, emtricitabine, and tenofovir alafenamide from dolutegravir plus abacavir and lamivudine in virologically suppressed adults with HIV-1: 48 week results of a randomised, double-blind, multicentre, active-controlled, phase 3, non-inferiority trial.Lancet HIV. 2018 Jul;5(7):e357-e365. doi: 10.1016/S2352-3018(18)30092-4. Epub 2018 Jun 18. Lancet HIV. 2018. PMID: 29925489 Clinical Trial.
-
An indirect comparison of 144-week efficacy, safety, and tolerability of dolutegravir plus lamivudine and second-generation integrase inhibitor-based, 3-drug, single-tablet regimens in therapy-naive people with HIV-1.AIDS Res Ther. 2023 Mar 22;20(1):17. doi: 10.1186/s12981-023-00507-1. AIDS Res Ther. 2023. PMID: 36949442 Free PMC article.
-
Abacavir/dolutegravir/lamivudine single-tablet regimen: a review of its use in HIV-1 infection.Drugs. 2015 Apr;75(5):503-14. doi: 10.1007/s40265-015-0361-6. Drugs. 2015. PMID: 25698454 Review.
References
-
- Ávila-Ríos S., García-Morales C., Valenzuela-Lara M., Chaillon A., Tapia-Trejo D., Pérez-García M., et al. HIV-1 drug resistance before initiation or re-initiation of first-line ART in eight regions of Mexico: a sub-nationally representative survey. J Antimicrob Chemother. 2019;74:1044–1055. doi: 10.1093/jac/dky512. - DOI - PMC - PubMed
-
- Ávila-Ríos S., García-Morales C., Matías-Florentino M., Romero-Mora K.A., Tapia-Trejo D., Quiroz-Morales V.S., et al. Pretreatment HIV-drug resistance in Mexico and its impact on the effectiveness of first-line antiretroviral therapy: a nationally representative 2015 WHO survey. Lancet HIV. 2016;3:e579–e591. doi: 10.1016/S2352-3018(16)30119-9. - DOI - PubMed
-
- TenoRes Study Group Global epidemiology of drug resistance after failure of WHO recommended first-line regimens for adult HIV-1 infection: a multicentre retrospective cohort study. Lancet Infect Dis. 2016;16:565–575. doi: 10.1016/S1473-3099(15)00536-8. Erratum in: Lancet Infect Dis. Erratum 2016;16:636. - DOI - PMC - PubMed
-
- Chaumont C., Bautista-Arredondo S., Calva J.J., Bahena-González R.I., Sánchez-Juárez G.H., González de Araujo-Muriel A., et al. Antiretroviral purchasing and prescription practices in Mexico: constraints, challenges and opportunities. Salud Publica Mex. 2015;57:s171–s182. doi: 10.21149/spm.v57s2.7606. - DOI - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous

