Trustworthiness assessment of published clinical trials: Literature review of domains and questions
- PMID: 40475114
- PMCID: PMC11795897
- DOI: 10.1002/cesm.12099
Trustworthiness assessment of published clinical trials: Literature review of domains and questions
Abstract
Background: Historically, peer reviewing has focused on the importance of research questions/hypotheses, appropriateness of research methods, risk of bias, and quality of writing. Until recently, the issues related to trustworthiness-including but not limited to plagiarism and fraud-have been largely neglected because of lack of awareness and lack of adequate tools/training. We set out to identify all relevant papers that have tackled the issue of trustworthiness assessment to identify key domains that have been suggested as an integral part of any such assessment.
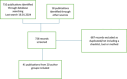
Methods: We searched the literature for publications of tools, checklists, or methods used or proposed for the assessment of trustworthiness of randomized trials. Data items (questions) were extracted from the included publications and transcribed on Excel including the assessment domain. Both authors then independently recategorised each data item in five domains (governance, plausibility, plagiarism, reporting, and statistics).
Results: From the 41 publications we extracted a total of 284 questions and framed 77 summary questions grouped in five domains: governance (13 questions), plausibility (17 questions), plagiarism (4 questions), reporting (29 questions), and statistics (14 questions).
Conclusion: The proposed menu of domains and questions should encourage peer reviewers, editors, systematic reviewers and developers of guidelines to engage in a more formal trustworthiness assessment. Methodologists should aim to identify the domains and questions that should be considered mandatory, those that are optional depending on the resources available, and those that could be discarded because of lack of discriminatory power.
© 2024 The Author(s). Cochrane Evidence Synthesis and Methods published by John Wiley & Sons Ltd on behalf of The Cochrane Collaboration.
Figures
Similar articles
-
Folic acid supplementation and malaria susceptibility and severity among people taking antifolate antimalarial drugs in endemic areas.Cochrane Database Syst Rev. 2022 Feb 1;2(2022):CD014217. doi: 10.1002/14651858.CD014217. Cochrane Database Syst Rev. 2022. PMID: 36321557 Free PMC article.
-
The future of Cochrane Neonatal.Early Hum Dev. 2020 Nov;150:105191. doi: 10.1016/j.earlhumdev.2020.105191. Epub 2020 Sep 12. Early Hum Dev. 2020. PMID: 33036834
-
The Effectiveness of Integrated Care Pathways for Adults and Children in Health Care Settings: A Systematic Review.JBI Libr Syst Rev. 2009;7(3):80-129. doi: 10.11124/01938924-200907030-00001. JBI Libr Syst Rev. 2009. PMID: 27820426
-
Checklist to assess Trustworthiness in RAndomised Controlled Trials (TRACT checklist): concept proposal and pilot.Res Integr Peer Rev. 2023 Jun 20;8(1):6. doi: 10.1186/s41073-023-00130-8. Res Integr Peer Rev. 2023. PMID: 37337220 Free PMC article.
-
Guidance for engagement in health guideline development: A scoping review.Campbell Syst Rev. 2024 Nov 25;20(4):e70006. doi: 10.1002/cl2.70006. eCollection 2024 Dec. Campbell Syst Rev. 2024. PMID: 39588485 Free PMC article.
Cited by
-
Data-sharing and trustworthiness of trials evaluating cervical ripening in induction of labour: a meta-epidemiological study of randomised controlled trials.EClinicalMedicine. 2025 Jul 8;85:103346. doi: 10.1016/j.eclinm.2025.103346. eCollection 2025 Jul. EClinicalMedicine. 2025. PMID: 40686691 Free PMC article.
References
-
- Smith R. Time to Assume That Health Research Is Fraudulent Until Proven Otherwise? The BMJ Opinion. 2021. https://blogs.bmj.com/bmj/2021/07/05/time-to-assume-that-health-research...
-
- Marcus A, Oransky I. Is there a retraction problem? And, if so, what can we do about it? In: Jamieson KH, ed. The Oxford Handbook of the Science of Science Communication. Oxford University Press; 2017:119‐126.
-
- Liverpool L. AI intensifies fight against ‘paper mills’ that churn out fake research. Nature. 2023;618:222‐223. - PubMed
Publication types
LinkOut - more resources
Full Text Sources