This is a preprint.
Understanding the pathogenesis of uveitis in Ebola virus disease survivors: a study protocol for clinical, molecular virologic, and immunologic characterization
- PMID: 40475131
- PMCID: PMC12140520
- DOI: 10.1101/2025.05.19.25327799
Understanding the pathogenesis of uveitis in Ebola virus disease survivors: a study protocol for clinical, molecular virologic, and immunologic characterization
Abstract
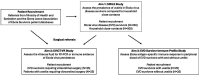
The 2013-2016 Western African outbreak of the Ebola virus disease (EVD), the largest recorded outbreak since the discovery of Ebola virus (EBOV) in 1976, destabilized local health systems and left thousands of survivors at risk for post-acute sequelae, including vision-threatening uveitis. In an EVD survivor with severe panuveitis, the detection of persistent EBOV in the aqueous humor, long after clearance of acute viremia, focused clinical and research attention on the host-EBOV interaction in the unique terrain of ocular immune-privilege. Despite the recognition that uveitis is common and consequential in EVD survivors, our understanding of pathogenesis is extremely limited, including the contributions of viral persistence and ocular-specific and systemic immune responses to disease expression. In this study protocol, we outline a multifaceted approach to characterize EVD-associated intraocular inflammation (EVD-IOI), including the clinical phenotype and complications; the presence of EBOV (or EBOV RNA/antigen) in ocular fluids and tissues; and associated local ocular-specific and peripheral immune responses. We utilize an observational cohort design, which includes EVD survivors and close contacts of EVD survivors (i.e., no documented history of EVD), and we propose disease (clinical examination and imaging), as well as molecular, virologic and immunologic characterization, to meet research objectives. Comprehensive findings emerging from the research will inform local stakeholders and global partners to understand and effectively address the individual and public health implications of EVD-associated uveitis, including to optimize clinical decision-making and medical intervention, identify potential ocular and peripheral biomarkers of viral persistence and ocular disease, and ensure effective infection prevention and control.
Figures

References
-
- Brett-Major DB. Severe emerging infections, survivorship, and the need for systematic approaches that incorporate clinical syndromes. Clin Infect Dis. 2021; 73: 1055–1057. - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
