This is a preprint.
FLAMeS: A Robust Deep Learning Model for Automated Multiple Sclerosis Lesion Segmentation
- PMID: 40475145
- PMCID: PMC12140514
- DOI: 10.1101/2025.05.19.25327707
FLAMeS: A Robust Deep Learning Model for Automated Multiple Sclerosis Lesion Segmentation
Abstract
Background and purpose: Assessment of brain lesions on MRI is crucial for research in multiple sclerosis (MS). Manual segmentation is time consuming and inconsistent. We aimed to develop an automated MS lesion segmentation algorithm for T2-weighted fluid-attenuated inversion recovery (FLAIR) MRI.
Methods: We developed FLAIR Lesion Analysis in Multiple Sclerosis (FLAMeS), a deep learning-based MS lesion segmentation algorithm based on the nnU-Net 3D full-resolution U-Net and trained on 668 FLAIR 1.5 and 3 tesla scans from persons with MS. FLAMeS was evaluated on three external datasets: MSSEG-2 (n=14), MSLesSeg (n=51), and a clinical cohort (n=10), and compared to SAMSEG, LST-LPA, and LST-AI. Performance was assessed qualitatively by two blinded experts and quantitatively by comparing automated and ground truth lesion masks using standard segmentation metrics.
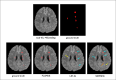
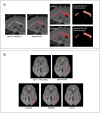
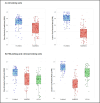
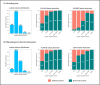
Results: In a blinded qualitative review of 20 scans, both raters selected FLAMeS as the most accurate segmentation in 15 cases, with one rater favoring FLAMeS in two additional cases. Across all testing datasets, FLAMeS achieved a mean Dice score of 0.74, a true positive rate of 0.84, and an F1 score of 0.78, consistently outperforming the benchmark methods. For other metrics, including positive predictive value, relative volume difference, and false positive rate, FLAMeS performed similarly or better than benchmark methods. Most lesions missed by FLAMeS were smaller than 10 mm3, whereas the benchmark methods missed larger lesions in addition to smaller ones.
Conclusions: FLAMeS is an accurate, robust method for MS lesion segmentation that outperforms other publicly available methods.
Conflict of interest statement
Dr. Reich has received research funding from Abata and Sanofi, unrelated to this paper. The other authors declare no competing financial interests.
Figures
Similar articles
-
Automated multiple sclerosis lesion segmentation from 3D-FLAIR MRI using R2AUNet: A deep learning approach with recurrent residual and attention mechanisms.Mult Scler Relat Disord. 2025 Jul 16;102:106620. doi: 10.1016/j.msard.2025.106620. Online ahead of print. Mult Scler Relat Disord. 2025. PMID: 40712506
-
Intracranial volume segmentation for neurodegenerative populations using multicentre FLAIR MRI.Neuroimage Rep. 2021 Mar 11;1(1):100006. doi: 10.1016/j.ynirp.2021.100006. eCollection 2021 Mar. Neuroimage Rep. 2021. PMID: 40568234 Free PMC article.
-
Automated segmentation of thoracic aortic lumen and vessel wall on three-dimensional bright- and black-blood magnetic resonance imaging using nnU-Net.J Cardiovasc Magn Reson. 2025 Jun 11;27(2):101923. doi: 10.1016/j.jocmr.2025.101923. Online ahead of print. J Cardiovasc Magn Reson. 2025. PMID: 40513884
-
Transformers for Neuroimage Segmentation: Scoping Review.J Med Internet Res. 2025 Jan 29;27:e57723. doi: 10.2196/57723. J Med Internet Res. 2025. PMID: 39879621 Free PMC article.
-
Assessing the comparative effects of interventions in COPD: a tutorial on network meta-analysis for clinicians.Respir Res. 2024 Dec 21;25(1):438. doi: 10.1186/s12931-024-03056-x. Respir Res. 2024. PMID: 39709425 Free PMC article. Review.
References
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous
