Immediately scheduled for an appointment to smoking cessation clinics: Key to quitting smoking in chronic airway disease - a multicenter randomized study
- PMID: 40475312
- PMCID: PMC12139391
- DOI: 10.18332/tid/204254
Immediately scheduled for an appointment to smoking cessation clinics: Key to quitting smoking in chronic airway disease - a multicenter randomized study
Abstract
Introduction: A significant proportion of patients with chronic airway diseases continue to smoke even after the diagnosis. In addition, smoking cessation support continues to be a neglected issue in real-life settings by physicians for that patient group. Therefore, in our search for a solution to this issue, we conducted our study to evaluate the effect of arranging immediate appointments to smoking cessation outpatient clinics on smoking cessation success in patients with chronic airway disease.
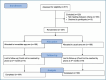
Methods: This multicenter, randomized, parallel-arm prospective study (NCT05764343) was conducted in pulmonary outpatient clinics between November 2022 and June 2023. Current smoker patients aged ≥18 years diagnosed with COPD, asthma, or bronchiectasis for at least 6 months were included and sequentially randomized in a 1:1 ratio. Both arms received brief smoking cessation interventions, and the intervention arm had immediate access to a smoking cessation clinic appointment. In contrast, the control arm received a standard quitline appointment for routine service. The primary endpoint was the self-reported smoking cessation rate at 3 months, analyzed using an intentionto-treat approach.
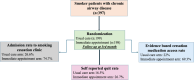
Results: The study comprised 198 patients in the immediate appointment arm and 199 in the usual care arm. The quit rate was significantly higher in the immediate appointment arm (26.7%) than in the usual care arm (16.5%, p=0.014). Access to smoking cessation medication was 69.3% in the intervention group against 22.0% in the control group (p<0.001). Multivariable analysis identified access to smoking cessation medication as the sole significant predictor of cessation success at 3 months (adjusted odds ratio, AOR=5.64; 95% CI: 2.89-11.03).
Conclusions: Our study revealed that access to evidence-based smoking cessation support is positively associated with successful quitting. Compared to the usual care arm, the immediately appointment-scheduled arm has a higher access rate of cessation support. Therefore, smoking cessation support, including pharmacotherapy, should be part of routine care for patients with chronic airway diseases.
Clinical trial registration: The study is registered on the official website of ClinicalTrials.gov Identifier: ID NCT05764343.
Keywords: lung diseases; obstructive; smoking cessation.
© 2025 Karadoğan D. et al.
Conflict of interest statement
The authors have completed and submitted the ICMJE Form for disclosure of Potential Conflicts of Interest and none was reported.
Figures
Similar articles
-
Effectiveness of immediate appointment scheduling in smoking cessation clinics for patients with chronic airway diseases: Preliminary results from a randomized trial.Tob Induc Dis. 2024 Aug 23;22. doi: 10.18332/tid/191782. eCollection 2024. Tob Induc Dis. 2024. PMID: 39184066 Free PMC article.
-
A bespoke smoking cessation service compared with treatment as usual for people with severe mental ill health: the SCIMITAR+ RCT.Health Technol Assess. 2019 Sep;23(50):1-116. doi: 10.3310/hta23500. Health Technol Assess. 2019. PMID: 31549622 Free PMC article. Clinical Trial.
-
Motivational support intervention to reduce smoking and increase physical activity in smokers not ready to quit: the TARS RCT.Health Technol Assess. 2023 Mar;27(4):1-277. doi: 10.3310/KLTG1447. Health Technol Assess. 2023. PMID: 37022933 Free PMC article.
-
Smoking reduction interventions for smoking cessation.Cochrane Database Syst Rev. 2019 Sep 30;9(9):CD013183. doi: 10.1002/14651858.CD013183.pub2. Cochrane Database Syst Rev. 2019. PMID: 31565800 Free PMC article.
-
A smoking cessation smartphone app that delivers real-time 'context aware' behavioural support: the Quit Sense feasibility RCT.Public Health Res (Southampt). 2024 Apr;12(4):1-99. doi: 10.3310/KQYT5412. Public Health Res (Southampt). 2024. PMID: 38676391 Clinical Trial.
Cited by
-
Prognostic Role of Inflammatory and Nutritional Indices in NSCLC Patients Treated with Immune Checkpoint Inhibitors: Retrospective, Multicenter, Turkish Oncology Group Study of Overall and Elderly Populations.Medicina (Kaunas). 2025 Jun 26;61(7):1160. doi: 10.3390/medicina61071160. Medicina (Kaunas). 2025. PMID: 40731790 Free PMC article.
References
Associated data
LinkOut - more resources
Full Text Sources
Medical
