This is a preprint.
Marginal zone and follicular B cells respond differently to TLR4 and TLR9 stimulation
- PMID: 40475402
- PMCID: PMC12139899
- DOI: 10.1101/2025.05.20.655194
Marginal zone and follicular B cells respond differently to TLR4 and TLR9 stimulation
Abstract
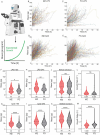
Marginal zone (MZ) B cells are considered to be innate-like immune cells, and follicular (FO) B cells are considered to be adaptive immune B cells. Yet, the proliferative response of MZ and FO B cells to different innate stimuli remains unclear. Here, we investigated cell growth, division, and death to determine the collective proliferative response of MZ and FO B cells in response to innate stimuli, LPS and CpG. We show that the growth rate of FO B cells is higher than that of MZ B cells in response to CpG, though MZ B cells acquire a higher mass at division, whereas both the growth rate and mass at division for MZ and FO B cells remain similar in response to LPS stimulation. We show that MZ B cells divide faster and induce a higher cRel expression in response to both CpG and LPS stimulation than FO B cells. A higher proportion of MZ B cells enter first division in response to LPS stimulation, not in response to CpG stimulation, than FO B cells. Interestingly, CpG stimulation, not LPS stimulation, leads to higher cell death in MZ B cells than FO B cells. In response to LPS stimulation, MZ B cells show a higher cell number at early time and a reduced/similar cell number at late time, whereas in response to CpG stimulation, MZ B cells show a lower cell number at both early and late time. Our study suggests that LPS and CpG stimulation impact cell growth, division, and death differently, which in turn regulate different proliferative responses of MZ and FO B cells. Thus, our study offers a new perspective that different innate stimuli regulate different features of proliferative responses.
Keywords: B cell; Toll-like receptor; microscopy; proliferation.
Conflict of interest statement
Conflicts of interest There are no conflicts of interest to declare.
Figures


References
-
- Pillai S., and Cariappa A.. 2009. The follicular versus marginal zone B lymphocyte cell fate decision. Nat Rev Immunol 9: 767–777. - PubMed
-
- Genestier L., Taillardet M., Mondiere P., Gheit H., Bella C., and Defrance T.. 2007. TLR agonists selectively promote terminal plasma cell differentiation of B cell subsets specialized in thymus-independent responses. J Immunol 178: 7779–7786. - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
