This is a preprint.
Long-term immune reprogramming of classical monocytes with altered ontogeny mediates enhanced lung injury in sepsis survivors
- PMID: 40475424
- PMCID: PMC12139851
- DOI: 10.1101/2025.05.16.654442
Long-term immune reprogramming of classical monocytes with altered ontogeny mediates enhanced lung injury in sepsis survivors
Abstract
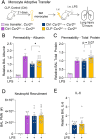
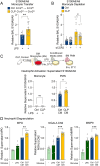
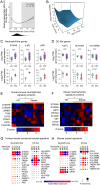
Patients who survive sepsis are predisposed to new hospitalizations for respiratory failure, but the underlying mechanisms are unknown. Using a murine model in which prior sepsis predisposes to enhanced lung injury, we previously discovered that classical monocytes persist in the lungs after long-term recovery from sepsis and exhibit enhanced cytokine expression after secondary challenge with intra-nasal lipopolysaccharide. Here, we hypothesized that immune reprogramming of post-sepsis monocytes and altered ontogeny predispose to enhanced lung injury. Monocyte depletion and/or adoptive transfer was performed three weeks and three months after sepsis. Monocytes from post-sepsis mice were necessary and sufficient for enhanced LPS-induced lung injury and promoted neutrophil degranulation. Prior sepsis enhanced JAK-STAT signaling and AP-1 binding in monocytes and shifted monocytes toward the neutrophil-like monocyte lineage. In human sepsis and/or pneumonia survivors, monocytes were predictive of 90-day mortality and exhibit transcriptional and proteomic neutrophil-like signatures. We conclude that sepsis reprograms monocytes into a pro-inflammatory phenotype and skews bone marrow progenitors and monocytes toward the neutrophil-like lineage, predisposing to neutrophil degranulation and lung injury.
Conflict of interest statement
Conflicts of Interest: A.I.N. is the founder of Fragmatics and serves on the scientific advisory boards of Protai Bio, Infinitopes, and Mobilion Systems.
Figures





References
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
