This is a preprint.
Ketogenic diet prevents obesity-associated pancreatic cancer independent of weight loss and induces pancreatic metabolic reprogramming
- PMID: 40475457
- PMCID: PMC12139956
- DOI: 10.1101/2025.05.20.655200
Ketogenic diet prevents obesity-associated pancreatic cancer independent of weight loss and induces pancreatic metabolic reprogramming
Abstract
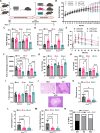
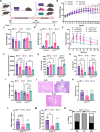
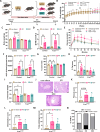
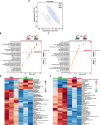
Pancreatic ductal adenocarcinoma (PDAC) is an aggressive cancer with poor outcomes. Obesity is a risk factor for several cancers including PDAC due to metabolic dysregulation and inflammation. The ketogenic diet (KD) can alter metabolism and has been evaluated for its effects on tumor progression in non-obese but not obese PDAC using genetically engineered mouse models (GEMMs). We hypothesized that ketone bodies and a KD alter cell and tumor metabolism. We show that ketone treatments altered pyrimidine metabolism in PDAC cells. Moreover, in an obese PDAC GEMM, KD prevented tumor progression independent of weight loss but promoted PDAC in a non-obese PDAC GEMM. The KD-specific delay of obesity-associated PDAC was associated with pancreatic metabolic shifts in pyrimidine, cysteine and methionine, and arginine and proline pathways. These findings suggest potential benefits of a KD in preventing obesity-associated PDAC, but highlights some risks in non-obese settings.
Conflict of interest statement
Declaration of interests JSV is cofounder and has equity in Virta Health, is a science advisor for Simply Good Foods, and has authored books on low-carbohydrate diets. The other authors declare no competing interests.
Figures

References
-
- Lee-Rueckert M., Canyelles M., Tondo M., Rotllan N., Kovanen P.T., Llorente-Cortes V., and Escolà-Gil J.C. (2023). Obesity-induced changes in cancer cells and their microenvironment: Mechanisms and therapeutic perspectives to manage dysregulated lipid metabolism. Semin Cancer Biol 93, 36–51. 10.1016/j.semcancer.2023.05.002. - DOI - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
