This is a preprint.
Glutamatergic Dysfunction of Astrocytes in Paraventricular Nucleus of Thalamus Contributes to Adult Anxiety Susceptibility in Adolescent Ethanol Exposed Mice
- PMID: 40475458
- PMCID: PMC12140005
- DOI: 10.1101/2025.05.20.654912
Glutamatergic Dysfunction of Astrocytes in Paraventricular Nucleus of Thalamus Contributes to Adult Anxiety Susceptibility in Adolescent Ethanol Exposed Mice
Abstract
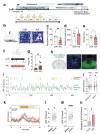
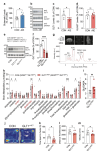
Repeated ethanol exposure during adolescence increases the risk for displaying anxiogenic phenotype in adulthood, but the underlying mechanisms are not fully understood. The paraventricular nucleus of thalamus (PVT) has been considered a hub brain area for controlling the anxiety network in the brain. Recent structural and functional investigations indicate that the PVT exhibits diverse neural signals aligned with early-life events, which are highly linked with anxiety-like behaviors. However, it remains unknown if repeated ethanol exposure during adolescence will affect the coordinated brain activities of the PVT in adulthood, and consequent behavioral adaptation. Here we show that adolescent repeated intermittent ethanol exposure (AIE) triggers anxiety-like behaviors and parallelly induces the glutamatergic adaptation in the PVT after four weeks withdrawal from the last ethanol exposure. The firing rates, along with the spatiotemporal calcium transients in the PVT neurons during behavior were increased in the AIE mice compared to those in the counterpart control mice. Importantly, with the chemogenetic inhibition of the PVT neurons, we found alleviated the anxiety-like behavior in the AIE mice. The increased neuronal activities in the PVT of AIE mice was, at least partly, via the reduction of GLT1 (an astrocyte dominant glutamate transporter, known as EAAT2, slc1a2). Our non-invasive magnetic resonance spectroscopy (MRS) measures also showed an increase in glutamate/GABA ratio in the thalamic area including the PVT of the GLT1 conditional knock-down mice, which exhibited the heightened anxiety-like behavior. In addition, while the selective knock-out of GLT1 in the astrocytes of PVT in the alcohol naïve mice induces anxiogenic phenotypes, the selective upregulation of GLT1 in the PVT astrocytes of the mice that were treated with AIE paradigm alleviated the anxiety-like behaviors as well. These findings highlight the significant role of PVT astrocytic GLT1 in the anxiogenic phenotype in adulthood induced by withdrawal from adolescent repeated ethanol exposure, suggesting that GLT1 in the PVT could serve as a therapeutic target for alcohol use disorder and comorbid emotional disorders.
Conflict of interest statement
Conflict of Interests All the authors declare that the research was conducted without any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures



Similar articles
-
Adolescent intermittent ethanol exposure induces sex-specific and time-dependent changes in affective behaviors and metabolomic profiles.bioRxiv [Preprint]. 2025 May 4:2025.04.29.651256. doi: 10.1101/2025.04.29.651256. bioRxiv. 2025. PMID: 40654638 Free PMC article. Preprint.
-
The Role of Paraventricular Nucleus of Thalamus in Sleep Disturbance Induced by Withdrawal from Repeated Ethanol Exposure.bioRxiv [Preprint]. 2025 Jun 8:2025.06.04.657945. doi: 10.1101/2025.06.04.657945. bioRxiv. 2025. PMID: 40501895 Free PMC article. Preprint.
-
Paraventricular Nucleus of the Thalamus Neurons That Project to the Nucleus Accumbens Show Enhanced c-Fos Expression During Early-Stage Cue-Reward Associative Learning in Male Rats.Eur J Neurosci. 2025 Jun;61(12):e70168. doi: 10.1111/ejn.70168. Eur J Neurosci. 2025. PMID: 40542605 Free PMC article.
-
Prevalence and odds of anxiety and depression in cutaneous malignant melanoma: a proportional meta-analysis and regression.Br J Dermatol. 2024 Jun 20;191(1):24-35. doi: 10.1093/bjd/ljae011. Br J Dermatol. 2024. PMID: 38197404
-
Assessing the comparative effects of interventions in COPD: a tutorial on network meta-analysis for clinicians.Respir Res. 2024 Dec 21;25(1):438. doi: 10.1186/s12931-024-03056-x. Respir Res. 2024. PMID: 39709425 Free PMC article. Review.
References
-
- Sawyer SM, Azzopardi PS, Wickremarathne D, Patton GC. The age of adolescence. Lancet Child Adolesc Health. 2018;2(3):223–28. - PubMed
-
- Schneider M. Adolescence as a vulnerable period to alter rodent behavior. Cell Tissue Res. 2013;354(1):99–106. - PubMed
-
- Radulescu CI, Cerar V, Haslehurst P, Kopanitsa M, Barnes SJ. The aging mouse brain: cognition, connectivity and calcium. Cell Calcium. 2021;94:102358. - PubMed
-
- White A, Hingson R. A Primer on Alcohol and Adolescent Brain Development: Implications for Prevention. In: Sloboda Z, Petras H, Robertson E, Hingson R, editors. Prevention of Substance Use. Cham: Springer International Publishing; 2019. p. 3–18.
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
