This is a preprint.
Single-Cell Epigenomics Uncovers Heterochromatin Instability and Transcription Factor Dysfunction during Mouse Brain Aging
- PMID: 40475496
- PMCID: PMC12139859
- DOI: 10.1101/2025.04.21.649585
Single-Cell Epigenomics Uncovers Heterochromatin Instability and Transcription Factor Dysfunction during Mouse Brain Aging
Abstract
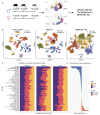
The mechanisms regulating transcriptional changes in brain aging remain poorly understood. Here, we use single-cell epigenomics to profile chromatin accessibility and gene expression across eight brain regions in the mouse brain at 2, 9, and 18 months of age. In addition to a significant decline in progenitor cell populations involved in neurogenesis and myelination, we observed widespread and concordant changes of transcription and chromatin accessibility during aging in glial and neuronal cell types. These alterations are accompanied by dysregulation of master transcription factors and a shift toward stress-responsive programs driven by AP-1, indicating a progressive loss of cell identity with aging. We also identify region- and cell-type-specific heterochromatin decay, characterized by increased accessibility at H3K9me3-marked domains, activation of transposable elements, and upregulation of long non-coding RNAs, particularly in glutamatergic neurons. Together, these results reveal age-related disruption of heterochromatin maintenance and transcriptional programs, identify vulnerable brain regions and cell types, and pinpoint key molecular pathways altered in brain aging.
Conflict of interest statement
B.R. is a co-founder and consultant of Arima Genomics, Inc., and a co-founder of Epigenome Technologies Inc.
Figures




References
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
