This is a preprint.
Anti-amyloid antibody equilibrium binding to Aβ aggregates from human Alzheimer disease brain
- PMID: 40475543
- PMCID: PMC12139811
- DOI: 10.1101/2025.05.20.654902
Anti-amyloid antibody equilibrium binding to Aβ aggregates from human Alzheimer disease brain
Abstract
Importance: Anti-amyloid immunotherapy is used to treat Alzheimer disease (AD) with moderate benefits and potentially serious side effects due to amyloid related imaging abnormality with effusions/edema (ARIA-E). Different anti-amyloid antibodies have different in vitro binding characteristics to different synthetic Aβ aggregates, leading to the assumption that they bind different species in the human brain. Lecanemab is hypothesized to bind "protofibrils," but these are not well-characterized in human brain. It is also unknown how binding differences correlate with ARIA-E rates. The APOE ε4 allele increases ARIA-E risk, but how it affects antibody binding characteristics is unknown.
Objectives: To determine whether anti-amyloid antibodies bind different species of human brain Aβ and whether these binding properties to human brain Aβ explains ARIA-E rates.
Design: Cross-sectional study of 18 postmortem human brains.
Setting: Single tertiary care hospital.
Participants: Deceased patients with AD and cerebral amyloid angiopathy (CAA).
Main outcomes and measures: Equilibrium binding constants (KD) and total Aβ binding (Bmax) of recombinant aducanumab, lecanemab, and donanemab equivalents to human brain soluble and insoluble amyloid plaque-enriched and CAA-enriched Aβ aggregates.
Results: Lecanemab did not bind with greater affinity to the soluble fraction of Aβ compared to aducanumab. All three antibodies were bound essentially identical quantities of Aβ across the 18 cases and fractions (Pearson's r 0.84 - 0.97). Antibody preference for plaque vs CAA Aβ did not differ in soluble fractions but differed slightly in insoluble extracts. The APOE ε4 allele led to a more soluble antibody-accessible Aβ pool in a dose-dependent manner for all three antibodies.
Conclusions and relevance: The lecanemab binding target in human brain is unlikely to be distinctly "protofibrillar" compared to other antibodies. Differences in antibody preference for plaque vs CAA Aβ are unlikely to fully explain differences in ARIA-E rates. The APOE ε4 allele may plausibly increase ARIA-E risk by making antibody-accessible Aβ more soluble. These results have implications for improving the safety and efficacy of current and future anti-amyloid antibody therapies.
Figures



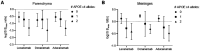
References
-
- Söderberg L., Johannesson M., Nygren P., Laudon H., Eriksson F., Osswald G., Möller C., and Lannfelt L. (2022). Lecanemab, Aducanumab, and Gantenerumab — Binding Profiles to Different Forms of Amyloid-Beta Might Explain Efficacy and Side Effects in Clinical Trials for Alzheimer’s Disease. Neurotherapeutics 20, 195. 10.1007/S13311-022-01308-6. - DOI - PMC - PubMed
-
- Solopova E., Romero-Fernandez W., Harmsen H., Ventura-Antunes L., Wang E., Shostak A., Maldonado J., Donahue M.J., Schultz D., Coyne T.M., et al. (2023). Fatal iatrogenic cerebral β-amyloid-related arteritis in a woman treated with lecanemab for Alzheimer’s disease. Nat Commun 14, 8220. 10.1038/S41467-023-43933-5. - DOI - PMC - PubMed
-
- Reish N.J., Jamshidi P., Stamm B., Flanagan M.E., Sugg E., Tang M., Donohue K.L., McCord M., Krumpelman C., Mesulam M.-M., et al. (2023). Multiple Cerebral Hemorrhages in a Patient Receiving Lecanemab and Treated with t-PA for Stroke. N Engl J Med 388, 478. 10.1056/NEJMC2215148. - DOI - PMC - PubMed
-
- Gkanatsiou E., Sahlin C., Portelius E., Johannesson M., Söderberg L., Fälting J., Basun H., Möller C., Odergren T., Zetterberg H., et al. (2021). Characterization of monomeric and soluble aggregated Aβ in Down’s syndrome and Alzheimer’s disease brains. Neurosci Lett 754, 135894. 10.1016/J.NEULET.2021.135894. - DOI - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous
