This is a preprint.
Targeting Regnase-1 unleashes CAR T cell antitumor activity for osteosarcoma and creates a proinflammatory tumor microenvironment
- PMID: 40475601
- PMCID: PMC12139752
- DOI: 10.1101/2025.05.20.650777
Targeting Regnase-1 unleashes CAR T cell antitumor activity for osteosarcoma and creates a proinflammatory tumor microenvironment
Abstract
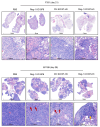
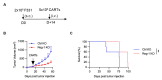
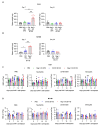
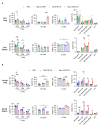
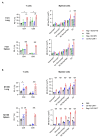
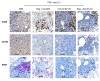
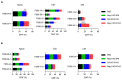
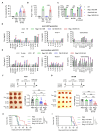
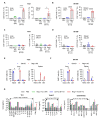
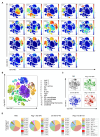
Negative regulators of T cell function represent promising targets to enhance the intrinsic antitumor activity of CAR T cells against solid tumors. However, the endogenous immune ecosystem in solid tumors often represents an immunosuppressive therapeutic barrier to CAR T cell therapy, and it is currently unknown whether deletion of negative regulators in CAR T cells reshapes the endogenous immune landscape. To address this knowledge gap, we developed CAR T cells targeting B7-H3 in immune-competent osteosarcoma models and evaluated the intrinsic and extrinsic effects of deleting a potent negative regulator called Regnase-1 (Reg-1). Deletion of Reg-1 not only improved the effector function of B7-H3-CAR T cells but also endowed them with the ability to create a proinflammatory landscape characterized by an influx of IFNγ-producing endogenous T cells and NK cells and a reduction of inhibitory myeloid cells, including M2 macrophages. Thus, deleting negative regulators in CAR T cells enforces a non-cell-autonomous state by creating a proinflammatory tumor microenvironment.
Conflict of interest statement
Conflict of Interest G.K., C.D., H.C., and S.G are co-inventors on patent applications or patents in the fields of cell or gene therapy for cancer. S.G. is a member of the Scientific Advisory Board of Be Biopharma and the Data and Safety Monitoring Board (DSMB) of Immatics and has received honoraria from CARGO Therapeutics within the last year. H.C. consults for Kumquat Biosciences and TCura Bioscience. All other authors do not declare a conflict of interest.
Figures







References
-
- Uslu U. & June C.H. Beyond the blood: expanding CAR T cell therapy to solid tumors. Nat Biotechnol (2024). - PubMed
-
- Albelda S.M. CAR T cell therapy for patients with solid tumours: key lessons to learn and unlearn. Nat Rev Clin Oncol 21, 47–66 (2024). - PubMed
-
- Del Bufalo F., et al. GD2-CART01 for Relapsed or Refractory High-Risk Neuroblastoma. N Engl J Med 388, 1284–1295 (2023). - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous
