This is a preprint.
Synaptic proteins that aggregate and degrade slower with aging accumulate in microglia
- PMID: 40475609
- PMCID: PMC12139995
- DOI: 10.1101/2025.05.20.654652
Synaptic proteins that aggregate and degrade slower with aging accumulate in microglia
Abstract
Neurodegenerative diseases affect 1 in 12 people globally and remain incurable. Central to their pathogenesis is a loss of neuronal protein maintenance and the accumulation of protein aggregates with aging1,2. We engineered bioorthogonal tools3 which allowed us to tag the nascent neuronal proteome and study its turnover with aging, its propensity to aggregate, and its interaction with microglia. We discovered neuronal proteins degraded on average twice as slowly between 4- and 24-month-old mice with individual protein stability differing between brain regions. Further, we describe the aged neuronal 'aggregome' encompassing 574 proteins, nearly 30% of which showed reduced degradation. The aggregome includes well-known proteins linked to disease as well as a trove of proteins previously not associated with neurodegeneration. Unexpectedly, we found 274 neuronal proteins accumulated in microglia with 65% also displaying reduced degradation and/or aggregation with age. Among these proteins, synaptic proteins were highly enriched, suggesting a cascade of events emanating from impaired synaptic protein turnover and aggregation to the disposal of these proteins, possibly by the engulfment of synapses by microglia. These findings reveal the dramatic loss of neuronal proteome maintenance with aging which could be causal for age-related synapse loss and cognitive decline.
Figures
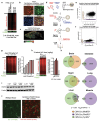
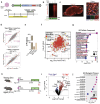
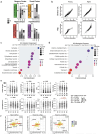

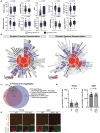





References
Methods References
-
- Isolation of mouse brain-infiltrating leukocytes for single cell profiling of epitopes and transcriptomes. https://star-protocols.cell.com/protocols/652. - PMC - PubMed
-
- FEAST: A flow cytometry-based toolkit for interrogating microglial engulfment of synaptic and myelin proteins ∣ Nature Communications. https://www.nature.com/articles/s41467-023-41448-7. - PMC - PubMed
-
- Radhiyanti P. T., Konno A., Matsuzaki Y. & Hirai H. Comparative study of neuron-specific promoters in mouse brain transduced by intravenously administered AAV-PHP.eB. Neurosci Lett 756, 135956 (2021). - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
