Comparative analysis of neutrophil dynamics and disease in SARS-CoV-2 Delta and Omicron variants utilizing an in vivo feline model for COVID-19
- PMID: 40475786
- PMCID: PMC12137312
- DOI: 10.3389/fimmu.2025.1547918
Comparative analysis of neutrophil dynamics and disease in SARS-CoV-2 Delta and Omicron variants utilizing an in vivo feline model for COVID-19
Abstract
Introduction: The emergence of SARS-CoV-2 variants, particularly Delta (B.1.617.2) and Omicron (XBB.1.5) variants, has substantially influenced the clinical and immunological landscape of COVID-19. This study investigates the differential pathogenicity and immune responses in a feline model infected with these variants, focusing on neutrophil activation, neutrophil extracellular trap (NET) formation, and cytokine profiles.
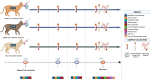
Methods: Eight pathogen-free cats were inoculated with B.1.617.2 (Delta) SARS-CoV-2 (n=3), XBB.1.5 (Omicron) SARS-CoV-2 (n=3), or vehicle (n=2), and clinical assessments, histopathological examinations, and cytokine analyses were performed post-infection.
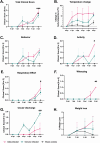
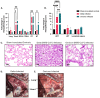
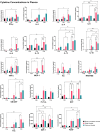
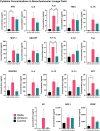
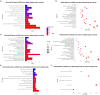
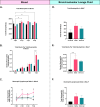
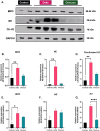
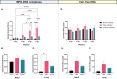
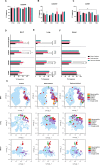
Results: Results demonstrate that Delta-infected cats exhibit more severe clinical manifestations characterized by significant elevation in respiratory effort, wheezing, and systemic inflammation compared to Omicron-infected cats, which show milder symptoms, primarily confined to the upper respiratory tract. Histopathological findings suggest pronounced lung damage in Delta-infected cats, whereas Omicron infection resulted in localized pathology. Cytokine profiling demonstrates heightened proinflammatory responses, particularly in Delta-infected cats, characterized by elevated levels of IL-6, IFN-γ and TNF-α while Omicron infection results in less pronounced inflammatory responses. Moreover, neutrophil-related parameters, including total neutrophil counts and banded neutrophils, were significantly elevated in Delta-infected cats, correlating with enhanced NET formation as evidenced by increased NETs-related markers MPO, NE, and citrullinated H3, and NET-specific markers MPO-DNA complexes and cell-free DNA.
Discussion: This study underscores the importance of variant-specific immune responses and highlights the need for targeted therapeutic strategies that mitigate severe lung injury associated with Delta infection, while also considering the distinct immune dynamics observed with the Omicron variant. Furthermore, results support the importance of delineating immune responses concerning future variants. These findings provide valuable insights into the pathogenesis of SARS-CoV-2 in companion animals and inform public health strategies as new variants continue to emerge.
Keywords: COVID-19; Delta; NETs; Omicron; SARS-CoV-2; feline; immunopathogenesis; neutrophils.
Copyright © 2025 Gunasekara, Shatnawi, More, Ludwig, Narayanan, Tamil Selvan, Miller and Rudd.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

References
-
- Aleem A, Ab AS, Slenker AK. Emerging variants of SARS-CoV-2 and novel therapeutics against coronavirus (COVID-19). (2021). - PubMed
Publication types
MeSH terms
Substances
Supplementary concepts
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

