Experimental procedures for studying microbial reactions under high hydrogen gas saturations in microcosms
- PMID: 40475891
- PMCID: PMC12139486
- DOI: 10.1016/j.mex.2025.103344
Experimental procedures for studying microbial reactions under high hydrogen gas saturations in microcosms
Abstract
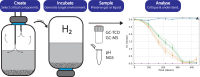
This methodology is proposed to investigate the response of microbial communities through analysis of headspace composition under high saturations of hydrogen. Changes in headspace composition will be related to specific communities and environmental conditions that will influence their response and result in changes in gases produced or potential changes in the liquid phase of microcosms pertaining to the hydrogen consumption rate through microbial metabolic processes. A step-by-step procedure is documented here.•Methodology includes an easy setup utilising common laboratory equipment.•The method showed minor appreciable loss of hydrogen from the microcosm setup/storage and the use of exetainers for gas measurements.•Actively studied microbial hydrogen consumption across 18 days at 30 °C and 50 °C This method is useful for the first instances in scientific studies towards understanding species or microbial communities found in environments with high percentages of hydrogen: underground hydrogen storage sites, hydrogen pipelines, and hydrogen leakage into subsurface soils.
Keywords: Acetogenesis; Hydrogen; Methanogenesis; Microbial metabolism; Microbiology studies under high saturations of hydrogen in headspace in microcosms; Microcosm studies; Sulfate reducing bacteria.
© 2025 The Author(s). Published by Elsevier B.V.
Conflict of interest statement
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures




Similar articles
-
Planning Implications Related to Sterilization-Sensitive Science Investigations Associated with Mars Sample Return (MSR).Astrobiology. 2022 Jun;22(S1):S112-S164. doi: 10.1089/AST.2021.0113. Epub 2022 May 19. Astrobiology. 2022. PMID: 34904892
-
Time-Sensitive Aspects of Mars Sample Return (MSR) Science.Astrobiology. 2022 Jun;22(S1):S81-S111. doi: 10.1089/AST.2021.0115. Epub 2022 May 19. Astrobiology. 2022. PMID: 34904889
-
Impact of organic acids and sulfate on the biogeochemical properties of soil from urban subsurface environments.J Environ Manage. 2021 Aug 15;292:112756. doi: 10.1016/j.jenvman.2021.112756. Epub 2021 May 25. J Environ Manage. 2021. PMID: 33984641
-
Artificial subsurface lithoautotrophic microbial ecosystems and gas storage in deep subsurface.FEMS Microbiol Ecol. 2024 Oct 25;100(11):fiae142. doi: 10.1093/femsec/fiae142. FEMS Microbiol Ecol. 2024. PMID: 39448371 Free PMC article. Review.
-
Upflow anaerobic sludge blanket reactor--a review.Indian J Environ Health. 2001 Apr;43(2):1-82. Indian J Environ Health. 2001. PMID: 12397675 Review.
References
-
- Verhoef H.A. The role of soil microcosms in the study of ecosystem processes. Ecology. 1996;77(3):685–690.
-
- Sugiura K. The use of an aquatic microcosm for pollution effects assessment. Water Res. 1996;30(8):1801–1812.
-
- Baars C., Jones T.Hefin, Edwards D. Microcosm studies of the role of land plants in elevating soil carbon dioxide and chemical weathering. Global Biogeochem. Cycles. 2008;22(3)
LinkOut - more resources
Full Text Sources

