Pre-sowing grain treatment with bio-AgNPs stimulates plant growth and affects redox homeostasis in maize
- PMID: 40475907
- PMCID: PMC12137326
- DOI: 10.3389/fpls.2025.1494741
Pre-sowing grain treatment with bio-AgNPs stimulates plant growth and affects redox homeostasis in maize
Abstract
Introduction: In the pursuit of sustainable development, nanotechnology provides effective solutions for enhancing agricultural productivity. Nanomaterials (NMs) can be effective in increasing plant abiotic and biotic stress tolerance. Understanding the nanoparticles (NPs)-plant interaction is essential to identify the potential of NPs for growth stimulation and phytotoxicity risks. Therefore, this study aimed to evaluate the effects of biologically synthesized silver nanoparticles (AgNPs) from Fusarium solani IOR 825 on the growth of Zea mays. Furthermore, the effect of AgNPs on oxidative stress and the antioxidant response was assessed.
Methods: AgNPs were efficiently synthesized from F. solani IOR 825 and characterized for physicochemical properties using transmission electron microscopy (TEM), nanoparticle tracking analysis (NTA), dynamic light scattering (DLS), X-ray diffraction (XRD), and Fourier transform infrared (FTIR) spectroscopy and measurement of Zeta potential. AgNPs at concentrations of 32, 128, and 512 µg mL-1 were used for the pre-sowing treatment of maize grains to inhibit microbial pathogens present on their surface. Sterilized maize grains were cultivated for 14 days for plantlet development. Subsequently, germination percentage (%G), mean germination time (MGT), germination rate index (GRI), fresh and dry weight (FW and DW), and the Ag content in plant organs and total chlorophyll content were analyzed. Hydrogen peroxide (H2O2) and malondialdehyde (MDA) were determined in leaves, roots, stems, and caryopses to assess the oxidative stress. The antioxidative system response to the AgNPs treatment was studied by determining total glutathione (GSH+GSSG) and ascorbate (ASC) contents as well as catalase (CAT), superoxide dismutase (SOD), peroxidase (POX), and ascorbate peroxidase (APX) activities.
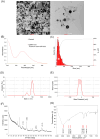
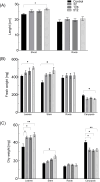
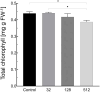
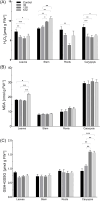
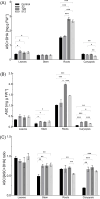
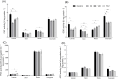
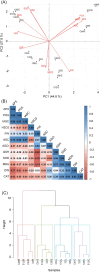
Results: AgNPs were spherical and small [TEM average diameter of 22.97 ± 9.4 nm, NTA average size of 43 ± 36 nm, and DLS average hydrodynamic diameters of 27.44 nm (14%) and 108.4 nm (86%)]. Zeta potential revealed that NPs were negatively charged [-19.5 mV (61.3%) and -2.93 mV (38.6%)]. The diffractogram of AgNPs confirmed the presence of a face-centered cubic structure of crystalline AgNPs, while FTIR spectra showed the presence of biomolecules on their surface. The results showed a dose-dependent effect on maize growth. The increase in length and fresh weight of plants treated with a AgNPs concentration of 512 µg mL-1 was noted. The treatment with all tested concentrations of AgNPs (32, 128, and 512 µg mL-1) resulted in increased dry weight of leaves. Reduced chlorophyll content was observed in plants treated with the highest tested concentration of AgNPs (512 µg mL-1). The treatment of grains with AgNPs decreased H2O2 levels in all organs, except the stem where the oxidant's level increased. MDA levels were unaffected except for the highest tested concentration of AgNPs, which raised its content in leaves. ASC and total glutathione levels were increased in roots and caryopses, respectively. The highest impact of AgNPs treatment was determined for SOD activity, which decreased in leaves, stems, and caryopses and increased in roots. CAT activity was decreased in leaves, stems, and roots. There was a minor effect on POX and APX activities.
Conclusion: The lowest tested concentration of AgNPs (32 µg mL-1) on maize efficiently inhibits maize-borne pathogens, without any negative effect on plant growth and chlorophyll content. Moreover, it does not provoke oxidative stress. However, AgNPs may affect cellular redox systems when their higher concentrations (128 and 512 µg mL-1) are used. The results indicate the potential use of biogenically synthesized AgNPs in agriculture through a crop-safe approach to eliminate pathogens and increase maize production efficiency.
Keywords: Zea mays; biogenic nanoparticles; crop protection; plant growth stimulators; seed priming.
Copyright © 2025 Trzcińska-Wencel, Mucha, Rai, Tyburski and Golińska.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
References
-
- Abbasi Khalaki M., Moameri M., Asgari Lajayer B., Astatkie T. (2021). Influence of nano-priming on seed germination and plant growth of forage and medicinal plants. Plant Growth Regul. 93, 13–28. doi: 10.1007/s10725-020-00670-9 - DOI
-
- Abdelgadir A., Adnan M., Patel M., Saxena J., Alam M. J., Alshahrani M. M., et al. (2024). Probiotic Lactobacillus salivarius mediated synthesis of silver nanoparticles (AgNPs-LS): A sustainable approach and multifaceted biomedical application. Heliyon 10, e37987. doi: 10.1016/j.heliyon.2024.e37987 - DOI - PMC - PubMed
-
- Abdul-Baki A. A., Anderson J. D. (1973). Vigor determination in soybean seed by multiple criteria 1. Crop Sci. 13, 630–633. doi: 10.2135/cropsci1973.0011183X001300060013x - DOI
-
- Abid N., Khan A. M., Shujait S., Chaudhary K., Ikram M., Imran M., et al. (2022). Synthesis of nanomaterials using various top-down and bottom-up approaches, influencing factors, advantages, and disadvantages: A review. Adv. Colloid Interface Sci. 300, 102597. doi: 10.1016/j.cis.2021.102597 - DOI - PubMed
-
- Acharya P., Jayaprakasha G. K., Crosby K. M., Jifon J. L., Patil B. S. (2019). Green-synthesized nanoparticles enhanced seedling growth, yield, and quality of onion (Allium cepa L.). ACS Sustain. Chem. Eng. 7, 14580–14590. doi: 10.1021/acssuschemeng.9b02180 - DOI
LinkOut - more resources
Full Text Sources
Miscellaneous

