Dietary modifications affect renal recovery during the healing phase following ischemic acute ischemic kidney injury
- PMID: 40476004
- PMCID: PMC12137256
- DOI: 10.3389/fcell.2025.1494660
Dietary modifications affect renal recovery during the healing phase following ischemic acute ischemic kidney injury
Abstract
Introduction: The effects of dietary modifications, such as varying amounts of salt, fat, and protein intake on the healing phase of acute kidney injury (AKI) remain to be elucidated. We investigated the effects of low- or high-salt/fat/protein diets on the intrarenal immunologic micromilieu and healing after renal ischemia-reperfusion injury (IRI) using murine ischemic AKI and human kidney-2 (HK-2) cell hypoxia models.
Methods: Three cohorts of male C57BL/6 mice (9-weeks old) were fed the designated diet from the third day following renal IRI until sacrifice (6 or 12 weeks after bilateral or unilateral IRI, respectively) in groups as follows: cohort 1, control, high- and low-salt/fat/protein; cohort 2, control, high- and low-salt; cohort 3, control, high- and low-fat/protein. Hypoxic HK-2 cells were treated with sodium chloride, amino acids, or fatty acids.
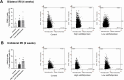
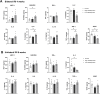
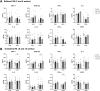
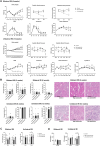
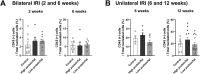
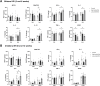
Results: Low-salt/fat/protein diet aggravated interstitial fibrosis, enhanced TGF-β expression, and induced more proinflammatory changes after bilateral IRI. High-salt diet aggravated renal tubular damage and enhanced the expression of intrarenal TGF-β after bilateral IRI, whereas low-salt diet enhanced the expression of intrarenal TGF-β after unilateral IRI. Low-salt diet induced more proinflammatory changes after bilateral IRI. Blood urea nitrogen levels were lower in the low fat/protein group than that in the control group following IRI. However, low-fat/protein diet aggravated interstitial fibrosis and enhanced intrarenal TGF-β expression after unilateral IRI. High sodium- or protein-containing media suppressed the proliferation of hypoxic HK-2 cells, whereas high lipid-containing media enhanced the proliferation of hypoxic HK-2 cells.
Conclusion: Excessive low or high salt, low fat, and low protein diet may adversely affect the healing process following renal IRI, supporting the importance of adequate and balanced nutrition during the recovery phase of ischemic AKI.
Keywords: acute kidney injury; diet; healing; immunologic micromilieu; ischemia-reperfusion injury.
Copyright © 2025 Jeon, Lee, Jeon, Yang, Lee, Lee, Kwon, Huh and Jang.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures







References
LinkOut - more resources
Full Text Sources

