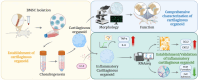
Establishment and characterization of an inflammatory cartilaginous organoids model for organoid transplantation study
- PMID: 40476067
- PMCID: PMC12138948
- DOI: 10.1016/j.jot.2025.05.002
Establishment and characterization of an inflammatory cartilaginous organoids model for organoid transplantation study
Abstract
Background: Transplantation of cartilaginous organoids for repairing cartilage defects in osteoarthritis represents a novel treatment approach. However, A controversial argument remains about whether cartilaginous organoids derived from the differentiation of bone marrow mesenchymal stem cells (BMSCs) in the three-dimensional (3D) environment are strictly organoids and whether the inflammatory microenvironment would affect the success rate of organoid transplantation. This study characterized 3D BMSC-derived cartilaginous organoids and developed an inflammatory organoid model to better understand the transcriptomic changes in the organoids induced by the microenvironment when transplanted into the knee with osteoarthritis.
Methods: Spatial growth BMSCs were generated and cultured in the cartilage differentiation medium to establish cartilaginous organoids. The model was characterized in both morphology and biology aspects. Subsequently, IL-1β induced inflammatory cartilaginous organoids were established and the transcriptomic sequencing was performed to investigate gene expression changes.
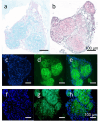
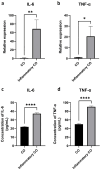
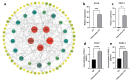
Results: BMSC-derived cartilaginous organoids were characterized by histology and immunofluorescence. Both Alcian blue and Safranin O staining revealed abundant articular cartilage extracellular matrix (ECM) in the organoids. The expression of cartilage specific ACAN and Col2A1 was confirmed by immunofluorescence. The organoids had the biological ability to repair cartilage defects. IL-1β induced inflammatory cartilaginous organoids were established and mRNA sequencing revealed downregulation of pathways related to cell adhesion and extracellular matrix organization. Upregulation of IL-6, TNF-α, CCL2 and CXCL1 was confirmed.
Conclusion: We thoroughly validated and characterized BMSC-derived cartilaginous organoids and established the inflammatory cartilaginous organoid models. This study revealed that the attenuation in cell adhesion and ECM formation of organoids induced by inflammatory chemokines may decrease the success rate and effectiveness of organoids auto-transplantation for fixing cartilage defects in the inflammatory microenvironment of the OA joint.
The translational potential of this article: By establishing and validating an in vitro inflammatory cartilaginous organoid model, this study provides a robust platform to examine how inflammatory mediators influence cartilage-like constructs. These findings enable the identification of targeted interventions to enhance the organoids' resilience against the inflammatory environment commonly found in osteoarthritic joints. Ultimately, this strategy offers a novel avenue for improving transplant success and promoting cartilage defect repair in patients with OA, thereby contributing valuable insights and potential clinical applications in regenerative medicine.
Keywords: Cartilaginous organoid; inflammation; transplantation.
© 2025 The Authors. Published by Elsevier B.V. on behalf of Chinese Speaking Orthopaedic Society.
Conflict of interest statement
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures



Similar articles
-
Senescence-targeted MicroRNA/Organoid composite hydrogel repair cartilage defect and prevention joint degeneration via improved chondrocyte homeostasis.Bioact Mater. 2024 May 29;39:427-442. doi: 10.1016/j.bioactmat.2024.05.036. eCollection 2024 Sep. Bioact Mater. 2024. PMID: 38855061 Free PMC article.
-
Articular Cartilage Repair with Mesenchymal Stem Cells After Chondrogenic Priming: A Pilot Study.Tissue Eng Part A. 2018 May;24(9-10):761-774. doi: 10.1089/ten.TEA.2017.0235. Epub 2017 Nov 30. Tissue Eng Part A. 2018. PMID: 28982297
-
Single BMSC-derived cartilage organoids for gradient heterogeneous osteochondral regeneration by leveraging native vascular microenvironment.J Nanobiotechnology. 2025 Apr 29;23(1):325. doi: 10.1186/s12951-025-03403-0. J Nanobiotechnology. 2025. PMID: 40301867 Free PMC article.
-
Small Joint Organoids 3D Bioprinting: Construction Strategy and Application.Small. 2024 Feb;20(8):e2302506. doi: 10.1002/smll.202302506. Epub 2023 Oct 9. Small. 2024. PMID: 37814373 Review.
-
Advancement of Organoid Technology in Regenerative Medicine.Regen Eng Transl Med. 2023;9(1):83-96. doi: 10.1007/s40883-022-00271-0. Epub 2022 Aug 8. Regen Eng Transl Med. 2023. PMID: 35968268 Free PMC article. Review.
Cited by
-
"Multidisciplinary synergy driving innovation in orthopaedic translational medicine".J Orthop Translat. 2025 Jun 4;52:A1-A3. doi: 10.1016/j.jot.2025.06.001. eCollection 2025 May. J Orthop Translat. 2025. PMID: 40698066 Free PMC article. No abstract available.
-
Construction of organoids using bioprinting technology: a frontier exploration of cartilage repair.J Orthop Translat. 2025 Jul 16;54:37-50. doi: 10.1016/j.jot.2025.06.020. eCollection 2025 Sep. J Orthop Translat. 2025. PMID: 40703568 Free PMC article. Review.
-
Applications in osteochondral organoids for osteoarthritis research: from pathomimetic modeling to tissue engineering repair.Front Bioeng Biotechnol. 2025 Jul 23;13:1629608. doi: 10.3389/fbioe.2025.1629608. eCollection 2025. Front Bioeng Biotechnol. 2025. PMID: 40771720 Free PMC article. Review.
References
-
- Manivong S., Cullier A., Audigié F., Banquy X., Moldovan F., Demoor M., et al. New trends for osteoarthritis: biomaterials, models and modeling. Drug Discov Today. 2023;28(3) - PubMed
-
- Salaris F., Rosa A. Construction of 3D in vitro models by bioprinting human pluripotent stem cells: challenges and opportunities. Brain Res. 2019;1723 - PubMed
LinkOut - more resources
Full Text Sources
Research Materials

