Use of O-arm to place an intrathecal catheter through a bony fusion mass: case report
- PMID: 40476074
- PMCID: PMC12137080
- DOI: 10.3389/fresc.2025.1530801
Use of O-arm to place an intrathecal catheter through a bony fusion mass: case report
Abstract
Background: Intrathecal baclofen (ITB) delivery is an FDA-approved indication for patients with intractable spasticity. Often, implantation in these patients can be considerably challenging, especially if previous surgical fusion involves the procedure access location.
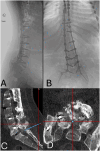
Case report: We present the case of a 27-year-old female with T2 American Spinal Injury Association (ASIA) A spinal cord injury (SCI) and chronic spastic dystonia. She was maximized on oral medications without satisfactory control of her painful muscle spasms and was a candidate for ITB trial, which ultimately failed due to the difficulty of accessing the spinal canal due to extensive pseudoarthrosis secondary to thoracic to lumbar fusion. A decision was made to directly implant the pump in the operative room using O-arm-aided neuronavigation to guide catheter access at L5-S1. Currently, at 22 months of follow-up post-pump implant, ITB delivery has led to persistent improvements in her spastic dystonia and many aspects of quality of life.
Discussion: The current case indicates that a multidisciplinary approach when considering surgical treatments for medication-refractory spasticity may help expand the indications to large numbers of patients with postsurgical spine abnormalities.
Keywords: O-arm; intrathecal baclofen; navigation; spasticity; spinal cord injury.
© 2025 Sammartino, Bavishi and Dalm.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
References
Publication types
LinkOut - more resources
Full Text Sources

