Molecular Design and Synthesis of Narrowband Near-Ultraviolet and Pure Deep-Blue Thermally Activated Delayed Fluorescence Materials by an Ether Group Strategy
- PMID: 40476312
- PMCID: PMC12322632
- DOI: 10.1002/anie.202510891
Molecular Design and Synthesis of Narrowband Near-Ultraviolet and Pure Deep-Blue Thermally Activated Delayed Fluorescence Materials by an Ether Group Strategy
Abstract
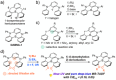
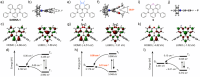
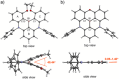
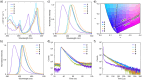
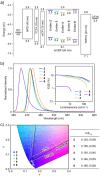
Boron-containing polycyclic aromatic hydrocarbons are promising materials for the development of displays due to their multiple-resonance thermally activated delayed fluorescence (MR-TADF) with narrowband emission. However, except for electrophilic aromatic borylation reactions, synthetic strategies for the generation of boron-containing MR-TADF molecules remain virtually unexplored. In particular, the synthesis of MR-TADF emitters that exhibit narrow near-ultraviolet and pure deep-blue emission constitutes a challenging task. Here, we present a directed tri-ortho-lithiation-borylation approach that provides a new family of N,N-bridge-type triphenylboranes that bear phenylimino groups instead of the methylene groups at the 8- and 14-positions and ether groups instead of the hydrogen atoms at the 3- and 19-positions of 1-borapentacyclohenicosanonaene. The effects of the electron-donating resonance of the oxygen atoms of the ether groups and the incorporation of oxygen atoms in the six-membered cycle allow the precise tuning of the HOMO-LUMO energy gaps, resulting in narrowband near-ultraviolet and pure deep-blue TADF with Commission-International-de-l'Éclairage coordinates (CIEx , y) of (0.142-0.160, 0.029-0.063) for the photoluminescence (PL) and (0.146-0.160, 0.026-0.053) for the electroluminescence (EL). The CIEx , y for the EL meet the BT.2020 requirement for the blue primary of ultrahigh-definition displays.
Keywords: Boron; Deep blue; Polycyclic aromatic hydrocarbons; Thermally activated delayed fluorescence; Ultraviolet.
© 2025 The Author(s). Angewandte Chemie International Edition published by Wiley‐VCH GmbH.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
-
- Anthony J. E., Chem. Rev. 2006, 106, 5028–5048. - PubMed
-
- Wöhrle T., Wurzbach I., Kirres J., Kostidou A., Kapernaum N., Litterscheidt J., Haenle J. C., Staffeld P., Baro A., Giesselmann F., Laschat S., Chem. Rev. 2016, 116, 1139–1241. - PubMed
-
- Stępień M., Gońka E., Żyła M., Sprutta N., Chem. Rev. 2017, 117, 3479–3716. - PubMed
-
- Janosik T., Rannug A., Rannug U., Wahlström N., Slätt J., Bergman J., Chem. Rev. 2018, 118, 9058–9128. - PubMed
-
- Hirai M., Tanaka N., Sakai M., Yamaguchi S., Chem. Rev. 2019, 119, 8291–8331, and references therein. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources

