Peripherin: A Novel Early Diagnostic and Prognostic Plasmatic Biomarker in Amyotrophic Lateral Sclerosis
- PMID: 40476320
- PMCID: PMC12142269
- DOI: 10.1111/ene.70241
Peripherin: A Novel Early Diagnostic and Prognostic Plasmatic Biomarker in Amyotrophic Lateral Sclerosis
Abstract
Background: Motor neuron diseases (MND) are heterogeneous and complex neurodegenerative disorders. Biomarkers could facilitate early diagnosis, prognosis determination, and patient stratification. Among the most studied biomarkers are neurofilaments, with peripherin (PRPH), a specific type predominantly expressed in the peripheral nervous system, gaining attention. To date, no studies have evaluated PRPH in human plasma.
Methods: Sandwich-ELISA was used to quantify plasma peripherin from 120 MND (100 ALS, 4 PMA, 15 PLS), 73 MND-mimics, and 38 healthy-controls (HCs). Plasma was collected at diagnosis or some months earlier. 41 ALS were evaluated longitudinally. ALSFRSr, MRC, spirometry, genetic tests, disease progression rate (PR), blood examinations, and neuropsychological tests were performed. Statistical analyses included Kruskal-Wallis, Mann-Whitney, Cox regression, and Kaplan-Meier curves.
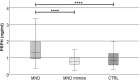
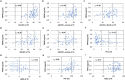
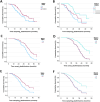
Results: Plasma PRPH levels differed significantly among groups (p < 0.0001), showing higher values in MND participants than MND mimics and HCs. Moreover, PRPH levels were elevated in PLS compared with HSP patients (p = 0.0001). Differences persisted after adjusting for age and sex. ROC curve demonstrated that PRPH discriminated MND from MND mimics (AUC = 0.85). Elevated PRPH correlated positively with ALSFRSr and lower motor neuron index, whereas inversely with disease progression rate. Higher PRPH levels at the beginning of the disease were associated with longer survival.
Discussion: Plasma PRPH is raised in MND, particularly ALS, from the earliest stages, distinguishing MND from mimics and correlating with clinical parameters and survival. This suggests PRPH may reflect an endogenous response of lower motor neuron to injury. Further multicenter studies are required to refine the diagnostic and prognostic utility of PRPH in MND.
Keywords: HSP; PLS; PRPH; fluid‐biomarker; mimics; neurodegeneration; neurofilament; regeneration.
© 2025 The Author(s). European Journal of Neurology published by John Wiley & Sons Ltd on behalf of European Academy of Neurology.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures

References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

