A Bird's-Eye View of Glycolytic Upregulation in Activated Brain: The Major Fate of Lactate Is Release From Activated Tissue, Not Shuttling to Nearby Neurons
- PMID: 40476345
- PMCID: PMC12142580
- DOI: 10.1111/jnc.70111
A Bird's-Eye View of Glycolytic Upregulation in Activated Brain: The Major Fate of Lactate Is Release From Activated Tissue, Not Shuttling to Nearby Neurons
Abstract
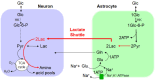
Glucose is the major, obligatory fuel for the brain, and nearly all glucose is oxidized in the awake, resting state. However, during activation, much of the glucose is not oxidized even though adequate oxygen is available, ATP demand is increased, and glycolysis generates less ATP than oxidation. The fate of the lactate produced by glycolysis is a highly debated topic, in part because its origin and fate in the living brain are difficult to measure. One idea has been that astrocytes generate lactate and shuttle it to neurons as a major fuel, but critical elements of the shuttle model are not validated, and there is no compelling evidence to support shuttling coupled with oxidation in vivo. Metabolic brain imaging reveals rapid loss of labeled metabolites of glucose from activated tissue that is mediated by lactate transporters and gap junctional trafficking among astrocytes. Lactate is highly labeled by [13C- and 14C]glucose, it is diffusible, and it is quickly released to blood and the perivascular-lymphatic drainage system. During intense sensory stimulation, astrocytic glycogen is consumed at half the rate of glucose by all brain cells; it is a major fuel. The oxygen-carbohydrate metabolic mismatch increases when glycogen is included in the calculation, revealing that glycogen is not oxidized. Although the energetics of brain activation is complex, metabolic modeling with comparison to a wide range of experimental data relating metabolism to neurotransmission strongly supports two concepts: (i) glycogenolysis in astrocytes spares blood-borne glucose for activated neurons, and (ii) the increase in cerebral blood flow in excess of oxygen consumption removes protons produced by glycolytic metabolism to maintain tissue pH, pO2, and pCO2 homeostasis. Several studies have identified processes and situations that involve neuronal aerobic glycolysis, and a better understanding of the roles of glycolysis in neuron-astrocyte interactions and functional metabolism in the normal and diseased brain is required.
Keywords: astrocyte; brain activation; glucose; glycogen; lactate; neuron.
© 2025 The Author(s). Journal of Neurochemistry published by John Wiley & Sons Ltd on behalf of International Society for Neurochemistry.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures



References
-
- Ackermann, R. F. , and Lear J. L.. 1989. “Glycolysis‐Induced Discordance Between Glucose Metabolic Rates Measured With Radiolabeled Fluorodeoxyglucose and Glucose.” Journal of Cerebral Blood Flow and Metabolism 9: 774–785. - PubMed
-
- Adachi, K. , Cruz N. F., Sokoloff L., and Dienel G. A.. 1995. “Labeling of Metabolic Pools by [6‐14C]Glucose During K(+)‐Induced Stimulation of Glucose Utilization in Rat Brain.” Journal of Cerebral Blood Flow and Metabolism 15: 97–110. - PubMed
-
- Almeida, A. , Moncada S., and Bolanos J. P.. 2004. “Nitric Oxide Switches on Glycolysis Through the AMP Protein Kinase and 6‐Phosphofructo‐2‐Kinase Pathway.” Nature Cell Biology 6: 45–51. - PubMed
-
- Anderson, C. M. , and Swanson R. A.. 2000. “Astrocyte Glutamate Transport: Review of Properties, Regulation, and Physiological Functions.” Glia 32: 1–14. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 NS087568/NH/NIH HHS/United States
- P41 EB027061/EB/NIBIB NIH HHS/United States
- R01 NS100106/NS/NINDS NIH HHS/United States
- P41 EB027061/NH/NIH HHS/United States
- R01 AG087526/AG/NIA NIH HHS/United States
- R01 MH109159/MH/NIMH NIH HHS/United States
- R01 NS087568/NS/NINDS NIH HHS/United States
- R56 AG079086/AG/NIA NIH HHS/United States
- R01 NS100106/NH/NIH HHS/United States
- R01 MH109159/NH/NIH HHS/United States
- DP1 AG093028/AG/NIA NIH HHS/United States
- DP1 AG093028/NH/NIH HHS/United States
- R56 AG079086/NH/NIH HHS/United States
- R01 AG087526/NH/NIH HHS/United States
LinkOut - more resources
Full Text Sources
Miscellaneous

