Comparison of Perceived Adverse Events After COVID-19 Vaccination Between Pregnant and NonPregnant Women Using Two Cohort Studies in the Netherlands
- PMID: 40476384
- PMCID: PMC12142568
- DOI: 10.1002/bdr2.2490
Comparison of Perceived Adverse Events After COVID-19 Vaccination Between Pregnant and NonPregnant Women Using Two Cohort Studies in the Netherlands
Abstract
Background: Maternal vaccines are upcoming. A clear picture of the adverse events (AEs) after maternal vaccination and whether this is comparable to a nonpregnant population is important. The objective of our study was to compare perceived AEs after COVID-19 vaccination between pregnant and nonpregnant women and to study if it is feasible to compare AEs within two independent Dutch cohort studies.
Methods: Data from the Dutch Pregnancy Drug Register (DPDR) and the cohort event monitoring (CEM) study on COVID-19 vaccines were used. At least one self-reported (solicited) AE, more than one AE, and specific self-reported AEs after the first doses of an mRNA COVID-19 vaccine were compared between pregnant and nonpregnant women using multivariable logistic regression analysis.
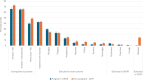
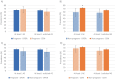
Results: The pattern of AEs was similar between pregnant (n = 2204) and nonpregnant (n = 2684) women, with the four most frequently reported AEs being: injection site reaction, myalgia, fatigue, and headache. Pregnant women reported less often at least one AE compared to nonpregnant women (65.9% vs. 72.3%; adjusted odds ratio [aOR] = 0.78; 95% confidence interval [CI] = 0.67-0.90), more than one AE, or specific AEs: nausea, chills, pyrexia, and arthralgia. Myalgia was more often reported among pregnant women compared to nonpregnant women.
Conclusions: Pregnant women perceived comparable or less often AEs after the first mRNA COVID-19 vaccination compared to nonpregnant women. The results aid pregnant women in making an informed decision about vaccination. A comparison between the pregnancy registry and the CEM study was feasible and this method can be used to compare AEs for other/future maternal vaccines.
Keywords: COVID‐19 vaccine; SARS‐CoV‐2; adverse events; cohort event monitoring; pregnancy.
© 2025 The Author(s). Birth Defects Research published by Wiley Periodicals LLC.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures

References
-
- Brinkley, E. , Mack C. D., Albert L., et al. 2022. “COVID‐19 Vaccinations in Pregnancy: Comparative Evaluation of Acute Side Effects and Self‐Reported Impact on Quality of Life Between Pregnant and Nonpregnant Women in the United States.” American Journal of Perinatology 39: 1750–1753. 10.1055/s-0042-1748158. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

