Advances in the discovery and study of Trichoderma natural products for biological control applications
- PMID: 40476465
- PMCID: PMC12142736
- DOI: 10.1039/d5np00017c
Advances in the discovery and study of Trichoderma natural products for biological control applications
Abstract
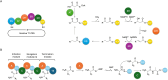
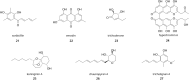
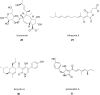
Covering: up to 2025Reducing the prevalence of phytopathogens and their impact on crops is essential to reach sustainable agriculture goals. Synthetic pesticides have been commonly used to control crop disease but are now strongly linked to disease resistance, environmental pollution, depletion of soil biodiversity, and bioaccumulation, leading to adverse effects on human health. As a alternative, the prolific Trichoderma genus has been studied for its biocontrol properties, as well as its ability to promote plant growth and increase nutrient uptake. This is done through various mechanisms, one of which is the production of bioactive natural products with high chemical diversity. These include terpenoids, alkaloids, non-ribosomal peptides, polyketides and RiPPs. One of the most studied examples is 6-pentyl-2H-pyran-2-one, a volatile organic polyketide, which induces systemic acquired resistance, morphogenesis, and natural product biosynthesis in plants. Methods for culturing Trichoderma spp., isolating and characterising unique bioactive metabolites are discussed here, with an emphasis on dereplication strategies using metabolomics to optimise discovery. In addition, the role of genome mining for the study of natural product biosynthesis in Trichoderma, and more generally, filamentous fungi is discussed. Examples of bioinformatics tools available to date are listed here with applications in Trichoderma and other ascomycetes. New advances in genome engineering in Trichoderma are also detailed, providing insights into available strategies for the validation of biosynthetic gene clusters identified using genome mining. Finally, the use of a combination of omics approaches, namely metabologenomics, is presented as a growing field for natural product discovery in fungi.
Conflict of interest statement
There are no conflicts to declare.
Figures





References
-
- Rani L. Thapa K. Kanojia N. Sharma N. Singh S. Grewal A. S. Srivastav A. L. Kaushal J. J. Cleaner Prod. 2021;283:124657. doi: 10.1016/j.jclepro.2020.124657. - DOI
-
- Lahdenperä M.-L., Simon E. and Uoti J., Developments in Agricultural and Managed Forest Ecology, Elsevier, 1991, vol. 23, pp. 258–263
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

