Hepatitis B Virus X Protein Upregulates SREBP2 to Modulate Autophagy in Hepatocellular Carcinoma
- PMID: 40476478
- PMCID: PMC12142432
- DOI: 10.1002/cam4.70916
Hepatitis B Virus X Protein Upregulates SREBP2 to Modulate Autophagy in Hepatocellular Carcinoma
Abstract
Background: The interaction between Hepatitis B virus X protein (HBx) and sterol regulatory element binding protein 2 (SREBP2) in modulating autophagy to influence inflammation and tumorigenesis is not fully understood. This research seeks to clarify the regulatory role of HBx in hepatocyte autophagy through SREBP2.
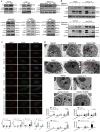
Methods: The study employed TCGA and GEO databases to investigate the expression of SREBF2 and autophagy-related proteins in liver cancer. Various experimental techniques, including dual-luciferase reporter assays, immunohistochemistry, Western blotting, immunofluorescence, GFP-mRFP-LC3 puncta analysis, transmission electron microscopy, and Fillipin III staining, were conducted on HBV-associated liver cancer tissues, HBV transgenic mice, and several liver cancer cell lines to assess the levels of HBx, SREBP2, autophagy, and cholesterol, respectively, as well as to explore potential associations between these factors.
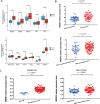
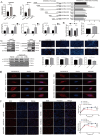
Results: Bioinformatics analysis suggested up-regulation of SREBP2 and autophagy-associated genes in HBV-associated liver cancer. Elevated levels of cholesterol, SREBP2, and autophagy flux were detected in HBV-associated liver cancer tissues as compared to adjacent tissues. HBV transgenic mice had higher cholesterol, SREBP2, and autophagy levels than wild-type mice. HBx activated the SREBP2 promoter to enhance its transcription and nuclear translocation. HBx knockdown down-regulated SREBP2 expression and nuclear translocation levels in HepG2.2.15-siHBx cells. HepG2.2.15 and HepG2-HBx showed more autolysosomes than HepG2 cells; furthermore, HepG2.2.15-siHBx cells had fewer autolysosomes than HepG2.2.15 cells.
Conclusions: This research highlights that HBx upregulates SREBP2 and increases autophagic flux, accompanied by changes in cholesterol metabolism, which offers an additional theoretical foundation to elucidate that chronic HBV infection causes abnormal lipid metabolism and induces tumorigenesis.
Keywords: Hepatitis B virus X protein; autophagy; cholesterol; hepatocellular carcinoma; sterol regulatory element binding protein 2.
© 2025 The Author(s). Cancer Medicine published by John Wiley & Sons Ltd.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures


References
-
- Martin‐Vilchez S., Lara‐Pezzi E., Trapero‐Marugán M., Moreno‐Otero R., and Sanz‐Cameno P., “The Molecular and Pathophysiological Implications of Hepatitis B X Antigen in Chronic Hepatitis B Virus Infection,” Reviews in Medical Virology 21, no. 5 (2011): 315–329. - PubMed
-
- Adugna A., “Histomolecular Characterisation of Hepatitis B Virus Induced Liver Cancer,” Reviews in Medical Virology 33, no. 6 (2023): e2485. - PubMed
MeSH terms
Substances
Grants and funding
- 2022J01730/the Fujian Provincial Natural Science Foundation of China
- 2023J01671/the Fujian Provincial Natural Science Foundation of China
- 2020J011025/the Fujian Provincial Natural Science Foundation of China
- 2020QNB019/the Fujian Provincial Health Technology Project
- 2021Y9064/the Innovation of Science and Technology
LinkOut - more resources
Full Text Sources
Medical

