Clinical Measurement of Transepidermal Water Loss
- PMID: 40476522
- PMCID: PMC12359141
- DOI: 10.1089/wound.2024.0148
Clinical Measurement of Transepidermal Water Loss
Abstract
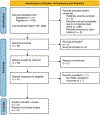
Significance: Recent reports recognize transepidermal water loss (TEWL) as a critical objective parameter measuring clinical wound healing endpoint. At the site of wound repair, TEWL measures functional wound closure as marked by re-establishment of barrier function at the wound site. This review article addresses recent developments in clinical TEWL measurement in the context of human skin health and wound care. To that end, emphasis is placed on the review of registered clinical studies reported in ClinicalTrials.gov for which TEWL results have been posted or published. Recent Advances: The U.S. Food and Drug Administration (FDA) defines complete wound closure as the achievement of 100% re-epithelialization of the wound surface, with no detectable exudate, drainage, or need for wound dressing, as verified during two sequential clinical assessments conducted at least 14 days apart. Clinically, wounds may meet this current FDA-recommended clinical criteria for wound closure, yet not achieve functional wound closure which requires the re-establishment of barrier function at the site of repair. Such wounds are likely to recur. High TEWL posthealing predicts wound recurrence. Thus, TEWL measurement at the site of repair posthealing is emerging as a significant measurement of wound healing endpoint. Critical Issues: Appropriate clinical measurement of TEWL requires a basic understanding of the related technologies and their appropriate use. Such understanding will help achieve the necessary rigor and reproducibility in clinical measurement. Future Directions: Recent reports on the critical significance of TEWL in wound care open new horizons wherein TEWL is likely to have broader applications involving altered skin barrier functions, such as during aging and other factors that determine skin health. Evidence to support revisiting the FDA definition of wound closure to include restoration of barrier function at the site of closure is strong. Widespread adoption of TEWL in wound care practices to determine functional wound closure is anticipated.
Keywords: TEWL; clinical trials; device; skin barrier function; transepidermal water loss.
Figures

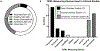

Similar articles
-
Prescription of Controlled Substances: Benefits and Risks.2025 Jul 6. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. 2025 Jul 6. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. PMID: 30726003 Free Books & Documents.
-
Negative pressure wound therapy for surgical wounds healing by primary closure.Cochrane Database Syst Rev. 2022 Apr 26;4(4):CD009261. doi: 10.1002/14651858.CD009261.pub7. Cochrane Database Syst Rev. 2022. PMID: 35471497 Free PMC article.
-
High Transepidermal Water Loss at the Site of Wound Closure Is Associated With Increased Recurrence of Diabetic Foot Ulcers: The NIDDK Diabetic Foot Consortium TEWL Study.Diabetes Care. 2025 Jul 1;48(7):1233-1240. doi: 10.2337/dc25-0300. Diabetes Care. 2025. PMID: 40445117 Free PMC article.
-
Negative pressure wound therapy for skin grafts and surgical wounds healing by primary intention.Cochrane Database Syst Rev. 2014 Oct 7;(10):CD009261. doi: 10.1002/14651858.CD009261.pub3. Cochrane Database Syst Rev. 2014. Update in: Cochrane Database Syst Rev. 2019 Mar 26;3:CD009261. doi: 10.1002/14651858.CD009261.pub4. PMID: 25287701 Updated.
-
The Black Book of Psychotropic Dosing and Monitoring.Psychopharmacol Bull. 2024 Jul 8;54(3):8-59. Psychopharmacol Bull. 2024. PMID: 38993656 Free PMC article. Review.
References
-
- U.S. FOOD AND DRUG ADMINISTRATION, Wound Healing Workshop. Available from: https://www.fda.gov/media/167142/download [Last accessed: March 10, 2025]. 2022.
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources

