Diagnostic performance of plasma p-tau217 and Aβ42/40 biomarkers in the outpatient memory clinic
- PMID: 40476540
- PMCID: PMC12142430
- DOI: 10.1002/alz.70316
Diagnostic performance of plasma p-tau217 and Aβ42/40 biomarkers in the outpatient memory clinic
Abstract
Introduction: Plasma biomarkers of Alzheimer's disease (AD) represent accessible alternatives to positron emission tomography (PET) and cerebrospinal fluid (CSF)-based biomarkers in well-characterized cohorts. It remains to be determined whether performance is maintained in outpatient clinics comprised of patients with multiple causes of cognitive impairment.
Methods: Plasma phosphorylated tau217 (p-tau217) and amyloid beta (Aβ) 42/40 were measured in 509 patients evaluated in a tertiary-care memory clinic. Biomarker performance was compared across diagnoses, referencing established cutpoints for positive and negative biomarkers.
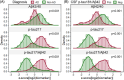
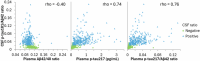
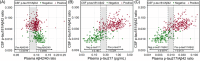
Results: Plasma p-tau217 distinguished patients with diagnoses of symptomatic AD with 95% sensitivity and 82% specificity. Integration of Aβ42 measurements (p-tau217/Aβ42) incrementally improved specificity (86%). Plasma p-tau217 and p-tau217/Aβ42 concentrations closely associated with established CSF biomarkers of AD (area under the curve [AUC]: 0.94 and 0.96, respectively). Reduced kidney function associated with elevated plasma p-tau217 and p-tau217/Aβ42 concentrations in patients without AD (referencing CSF biomarkers).
Discussion: Plasma p-tau217 and p-tau217/Aβ42 concentrations demonstrated robust diagnostic performance in a heterogeneous clinical cohort; interpretation requires consideration of kidney function.
Highlights: Plasma phosphorylated tau217 (p-tau217) reliably identified clinic patients with Alzheimer's disease. A two-cutpoint strategy yielded definitive results in 86% of patients. Integration of plasma amyloid beta (Aβ; p-tau217/Aβ42) minimally improved diagnostic performance. Plasma p-tau217 levels were increased in patients with kidney dysfunction.
Keywords: Alzheimer's disease; cerebrospinal fluid; dementia; diagnosis; kidney function; memory clinic; plasma biomarker.
© 2025 The Author(s). Alzheimer's & Dementia published by Wiley Periodicals LLC on behalf of Alzheimer's Association.
Conflict of interest statement
Y.D. Piura reports no conflicts of interest. D.J. Figdore reports no conflicts of interest. C. Lachner reports no conflicts of interest. His research is supported by the NIH (UH2‐AG083186, ARDC AG062677 Clinical Core, AG075802, NS125417). J. Bornhorst has participated on an advisory board for Sunbird Bio and received an honorarium from Roche Diagnostics. A. Algeciras‐Schimnich has participated on advisory boards for Roche Diagnostics and Fujirebio Diagnostis, and has received a honorarium from Roche Diagnostics. N.R. Graff‐Radford reports no conflicts of interest. His research is supported by NIH. He has participated in multicenter therapy studies sponsored by Biogen, Eisai and Lilly. G.S. Day reports no conflicts of interest. His research is supported by NIH (R01AG089380, U01AG057195, U01NS120901, U19AG032438). He serves as a consultant for Arialys Therapeutics and Parabon Nanolabs Inc, and as a Topic Editor (Dementia) for DynaMed (EBSCO). He is a co‐Project PI for a clinical trial in anti‐NMDAR encephalitis, which receives support from NINDS (U01NS120901) and Amgen Pharmaceuticals. He has developed educational materials for Continuing Education Inc and Ionis Pharmaceuticals. He owns stock in ANI pharmaceuticals. Dr. Day's institution has received in‐kind contributions for radiotracer precursors for tau‐PET neuroimaging in studies of memory and aging (via Avid Radiopharmaceuticals, a wholly owned subsidiary of Eli Lilly). Author disclosures are available in the supporting information.
Figures


References
-
- Olsson B, Lautner R, Andreasson U, et al. CSF and blood biomarkers for the diagnosis of Alzheimer's disease: a systematic review and meta‐analysis. The Lancet Neurology. 2016;15(7):673‐684. - PubMed
-
- Wu L, Rosa‐Neto P, Gauthier S. Use of biomarkers in clinical trials of Alzheimer disease: from concept to application. Molecular Diagnosis & Therapy. 2011;15:313‐325. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

