Mixed-Metal Ce-Zr-Mn Clusters as Photo-Catalysts for Decarboxylative Functionalization of Carboxylic Acids
- PMID: 40476749
- PMCID: PMC12338407
- DOI: 10.1002/anie.202505639
Mixed-Metal Ce-Zr-Mn Clusters as Photo-Catalysts for Decarboxylative Functionalization of Carboxylic Acids
Abstract
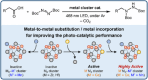
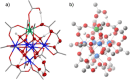
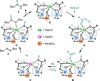
Decarboxylative hydrazination of carboxylic acids was achieved using a 1:5:2 ratio of three metal salts, Ce(OtBu)4, Zr(OtBu)4, and Mn(OAc)3, as a catalyst under visible light irradiation. The catalytic activity, compared with our previously developed Ce6 cluster photo-catalysts, was enhanced by the formation of single cerium-incorporated hexanuclear mixed-metal clusters containing a [CeZr5O4(OH)4]12+ core. The manganese salts further accelerated the overall reaction rate (10 times faster reaction rate with the manganese salt than that of the manganese-free conditions). Using the isolated cluster, CeZr5O4(OH)4(OCOCH2 tBu)12(HOCOCH2 tBu)4 (4a), with Mn(OAc)3, phenol and thiophenol-containing carboxylic acids were transformed to their decarboxylative hydrazinated products in moderate to high yields, while a mixture of Ce6O4(OH)4(OCOCH2 tBu)12(HOCOCH2 tBu)4 (4c) and Mn(OAc)3 or Ce(OtBu)4, Zr(OtBu)4, and Mn(OAc)3 yielded lower amounts of the products. These findings highlight the importance of incorporating cerium(IV) into the zirconium-based core to tolerate these easily oxidizable functional groups. Upon exposure of 4a to blue LED light under an argon atmosphere, the CeZr5 cluster produced 2,2,5,5-tetramethylhexane, a radical coupling product derived from the carboxylate ligand on 4a, in half an equivalent per cluster, consistent with the photo-reduction of cerium(IV) and inertness of the oxo- and hydroxo-bridged Zr5 motif as a metallo-ligand around the cerium(IV) site. Moreover, decarboxylative oxygenation of carboxylic acids under air followed by treatment with NaBH4 resulted in the production of one-carbon shortened alcohols in excellent yields when using Ce(OtBu)4 and Zr(OtBu)4 or Hf(OtBu)4 in a 1:5 ratio: the reaction rates were 8-10 times higher than that of the previously developed cerium-catalyzed reaction under identical conditions.
Keywords: Carboxylic acid; Cerium; Manganese; Zirconium.
© 2025 The Author(s). Angewandte Chemie International Edition published by Wiley‐VCH GmbH.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

Similar articles
-
Comparison of Two Modern Survival Prediction Tools, SORG-MLA and METSSS, in Patients With Symptomatic Long-bone Metastases Who Underwent Local Treatment With Surgery Followed by Radiotherapy and With Radiotherapy Alone.Clin Orthop Relat Res. 2024 Dec 1;482(12):2193-2208. doi: 10.1097/CORR.0000000000003185. Epub 2024 Jul 23. Clin Orthop Relat Res. 2024. PMID: 39051924
-
Intravenous magnesium sulphate and sotalol for prevention of atrial fibrillation after coronary artery bypass surgery: a systematic review and economic evaluation.Health Technol Assess. 2008 Jun;12(28):iii-iv, ix-95. doi: 10.3310/hta12280. Health Technol Assess. 2008. PMID: 18547499
-
Sexual Harassment and Prevention Training.2024 Mar 29. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. 2024 Mar 29. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. PMID: 36508513 Free Books & Documents.
-
The Black Book of Psychotropic Dosing and Monitoring.Psychopharmacol Bull. 2024 Jul 8;54(3):8-59. Psychopharmacol Bull. 2024. PMID: 38993656 Free PMC article. Review.
-
Cerium(IV) Carboxylate Photocatalyst for Catalytic Radical Formation from Carboxylic Acids: Decarboxylative Oxygenation of Aliphatic Carboxylic Acids and Lactonization of Aromatic Carboxylic Acids.J Am Chem Soc. 2020 Mar 25;142(12):5668-5675. doi: 10.1021/jacs.9b12918. Epub 2020 Mar 11. J Am Chem Soc. 2020. PMID: 32109060
References
-
- Romero N. A., Nicewicz D. A., Chem. Rev. 2016, 116, 10075–10166. - PubMed
-
- Twilton J., Le C., Zhang P., Shaw M. H., Evans R. W., MacMillan D. W. C., Nat. Rev. Chem. 2017, 1, 1–19.
Grants and funding
- JP20H02742/JSPS KAKENHI, Grant-in-Aid for Scientific Research(B)
- JP24K01486/JSPS KAKENHI, Grant-in-Aid for Scientific Research(B)
- JP21K01486/JSPS KAKENHI, Grant-in-Aid for Challenging Research (Exploratory)
- JP24H01853/Green Catalysis Science for Renovating Transformation of Carbon-Based Resources
- JP23H04906/Green Catalysis Science for Renovating Transformation of Carbon-Based Resources
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

