Deep Learning Reveals Liver MRI Features Associated With PNPLA3 I148M in Steatotic Liver Disease
- PMID: 40478199
- PMCID: PMC12143367
- DOI: 10.1111/liv.70164
Deep Learning Reveals Liver MRI Features Associated With PNPLA3 I148M in Steatotic Liver Disease
Abstract
Background: Steatotic liver disease (SLD) is the most common liver disease worldwide, affecting 30% of the global population. It is strongly associated with the interplay of genetic and lifestyle-related risk factors. The genetic variant accounting for the largest fraction of SLD heritability is PNPLA3 I148M, which is carried by 23% of the western population and increases the risk of SLD two to three-fold. However, identification of variant carriers is not part of routine clinical care and prevents patients from receiving personalised care.
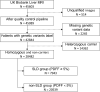
Methods: We analysed MRI images and common genetic variants in PNPLA3, TM6SF2, MTARC1, HSD17B13 and GCKR from a cohort of 45 603 individuals from the UK Biobank. Proton density fat fraction (PDFF) maps were generated using a water-fat separation toolbox, applied to the magnitude and phase MRI data. The liver region was segmented using a U-Net model trained on 600 manually segmented ground truth images. The resulting liver masks and PDFF maps were subsequently used to calculate liver PDFF values. Individuals with (PDFF ≥ 5%) and without SLD (PDFF < 5%) were selected as the study cohort and used to train and test a Vision Transformer classification model with five-fold cross validation. We aimed to differentiate individuals who are homozygous for the PNPLA3 I148M variant from non-carriers, as evaluated by the area under the receiver operating characteristic curve (AUROC). To ensure a clear genetic contrast, all heterozygous individuals were excluded. To interpret our model, we generated attention maps that highlight the regions that are most predictive of the outcomes.
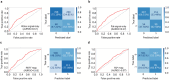
Results: Homozygosity for the PNPLA3 I148M variant demonstrated the best predictive performance among five variants with AUROC of 0.68 (95% CI: 0.64-0.73) in SLD patients and 0.57 (95% CI: 0.52-0.61) in non-SLD patients. The AUROCs for the other SNPs ranged from 0.54 to 0.57 in SLD patients and from 0.52 to 0.54 in non-SLD patients. The predictive performance was generally higher in SLD patients compared to non-SLD patients. Attention maps for PNPLA3 I148M carriers showed that fat deposition in regions adjacent to the hepatic vessels, near the liver hilum, plays an important role in predicting the presence of the I148M variant.
Conclusion: Our study marks novel progress in the non-invasive detection of homozygosity for PNPLA3 I148M through the application of deep learning models on MRI images. Our findings suggest that PNPLA3 I148M might affect the liver fat distribution and could be used to predict the presence of PNPLA3 variants in patients with fatty liver. The findings of this research have the potential to be integrated into standard clinical practice, particularly when combined with clinical and biochemical data from other modalities to increase accuracy, enabling easier identification of at-risk individuals and facilitating the development of tailored interventions for PNPLA3 I148M-associated liver disease.
Keywords: deep learning; medical imaging process; single nucleotide polymorphism; steatotic liver disease.
© 2025 The Author(s). Liver International published by John Wiley & Sons Ltd.
Conflict of interest statement
R.L. serves as a consultant to Aardvark Therapeutics, Altimmune, Arrowhead Pharmaceuticals, AstraZeneca, Cascade Pharmaceuticals, Eli Lilly, Gilead, Glympse bio, Inipharma, Intercept, Inventiva, Ionis, Janssen Inc., Lipidio, Madrigal, Neurobo, Novo Nordisk, Merck, Pfizer, Sagimet, 89 bio, Takeda, Terns Pharmaceuticals and Viking Therapeutics. R.L. has stock options in Sagimet biosciences. In addition, his institution received research grants from Arrowhead Pharmaceuticals, Astrazeneca, Boehringer‐Ingelheim, Bristol‐Myers Squibb, Eli Lilly, Galectin Therapeutics, Gilead, Intercept, Hanmi, Intercept, Inventiva, Ionis, Janssen, Madrigal Pharmaceuticals, Merck, Novo Nordisk, Pfizer, Sonic Incytes and Terns Pharmaceuticals. Co‐founder of LipoNexus Inc. J.N.K. declares consulting services for Bioptimus, France; Panakeia, UK; AstraZeneca, UK; and MultiplexDx, Slovakia. Furthermore, he holds shares in StratifAI, Germany, Synagen, Germany, and Ignition Lab, Germany; has received an institutional research grant by GSK; and has received honoraria by AstraZeneca, Bayer, Daiichi Sankyo, Eisai, Janssen, Merck, MSD, BMS, Roche, Pfizer, and Fresenius. L.V. declares speaking for: Viatris, Novo Nordisk, GSK, consulting for: Novo Nordisk, Pfizer, Boehringer Ingelheim, Resalis, MSD. All other authors declare no financial or non‐financial competing interests.
Figures


References
-
- Israelsen M., Francque S., Tsochatzis E. A., and Krag A., “Steatotic Liver Disease,” Lancet (London, England) 404, no. 10464 (2024): 1761–1778. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

