The mRNA-binding protein HLN1 enhances drought stress tolerance by stabilizing the GAD2 mRNA in Arabidopsis
- PMID: 40478352
- PMCID: PMC12144001
- DOI: 10.1007/s44154-025-00239-4
The mRNA-binding protein HLN1 enhances drought stress tolerance by stabilizing the GAD2 mRNA in Arabidopsis
Abstract
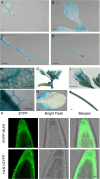
Drought is a common environmental condition that significantly impairs plant growth. In response to drought, plants close their stomata to minimize transpiration and meanwhile activate many stress-responsive genes to mitigate damage. These stress-related mRNA transcripts require the assistance of RNA-binding proteins throughout their metabolic process, culminating in protein synthesis in the cytoplasm. In this study, we identified HLN1 (Hyaluronan 1), an RNA-binding protein with similarity to the animal hyaluronan-binding protein 4 / serpin mRNA binding protein 1 (HABP4/SERBP1), as crucial for plant drought tolerance. The hln1 loss-of-function mutant exhibited higher transpiration rates due to impaired stomatal closure, making it highly susceptible to drought. Drought stress increased HLN1 expression, and the protein underwent liquid-liquid phase separation (LLPS) to form mRNA-ribonucleoprotein (mRNP) condensates in the cytoplasm under osmotic stress. We identified GAD2 as a potential mRNA target of HLN1. GAD2 encodes the predominant glutamate decarboxylase synthesizing γ-aminobutyric acid (GABA), a non-proteinogenic amino acid that modulates stomatal movement. RIP-qPCR and EMSA showed that HLN1 binds GAD2 mRNA, which promotes HLN1 condensate formation. In hln1 mutants, GAD2 transcripts were less stable, reducing steady-state mRNA levels. As a result, hln1 accumulated less GABA and exhibited impaired stomatal closure under drought. Conversely, HLN1 overexpression stabilized GAD2 mRNA, increased GABA levels, and enhanced drought tolerance in transgenic plants. GAD2 overexpression in hln1 mutants also rescued the drought-sensitive phenotypes. Overall, our study reveals a mechanism whereby HLN1 stabilizes GAD2 mRNA to enhance GABA production and drought tolerance. These findings provide novel strategies for engineering drought-resistant crops.
Keywords: Condensate; Drought; GABA; HLN1; mRNA stability; mRNA-binding protein.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Ethics approval and consent to participate: Not applicable. Consent for publication: All authors have read and approved the manuscript's content and consent to its publication in Stress Biology upon acceptance. Competing interests: L.X. is a member of the editorial board but was not involved in the journal's review, or any decisions, related to this submission.
Figures






References
-
- Bleckmann A, Spitzlberger N, Denninger P, Ehrnsberger HF, Wang L, Bruckmann A, Reich S, Holzinger P, Medenbach J, Grasser KD, Dresselhaus T (2023) Cytosolic RGG RNA-binding proteins are temperature sensitive flowering time regulators in Arabidopsis. Biol Chem 404(11–12):1069–1084. 10.1515/hsz-2023-0171 - DOI - PubMed
-
- Bounedjah O, Desforges B, Wu TD, Pioche-Durieu C, Marco S, Hamon L, Curmi PA, Guerquin-Kern JL, Piétrement O, Pastré D (2014) Free mRNA in excess upon polysome dissociation is a scaffold for protein multimerization to form stress granules. Nucleic Acids Res 42(13):8678–8691. 10.1093/nar/gku582 - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

