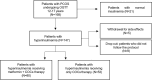
Sustained Metabolic Improvements with Low-Dose Metformin Combined with Oral Contraceptives in Female Adolescents with PCOS: A Single-Center Retrospective Cohort Study
- PMID: 40478377
- PMCID: PMC12313771
- DOI: 10.1007/s12325-025-03251-2
Sustained Metabolic Improvements with Low-Dose Metformin Combined with Oral Contraceptives in Female Adolescents with PCOS: A Single-Center Retrospective Cohort Study
Abstract
Introduction: This study aimed to evaluate metformin treatment's immediate and long-term efficacy in adolescent patients with polycystic ovary syndrome (PCOS) and hyperinsulinemia and the subsequent metabolic evolution post-treatment discontinuation.
Methods: This single-center, retrospective cohort study included 168 adolescent girls (12-17 years) diagnosed with PCOS between December 2018 and August 2024. All participants underwent an oral glucose tolerance test to evaluate insulin sensitivity and were stratified into two groups: patients with normal insulinemia (n = 21) and patients with hyperinsulinemia (n = 147). Patients with hyperinsulinemia were offered low-dose metformin (500 mg twice daily); 80 accepted and formed the treatment arm, while 53 declined and served as controls. Simultaneously, every subject received a continuous regimen of combined oral contraceptives (COCs) (30 µg ethinyl estradiol/3 mg drospirenone). Clinical, biochemical, and ultrasound assessments were conducted at baseline, at regular intervals during therapy, at the end of treatment, and at least 24 months after metformin discontinuation to evaluate immediate and long-term outcomes.
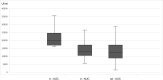
Results: Metformin therapy yielded favorable body mass index, insulin sensitivity, and androgenic profile outcomes. Remarkably, these benefits persisted beyond the cessation of treatment. Metformin responders showed a ≥ 20% decrease in the insulin area under the curve values post-treatment. Our investigation revealed a substantial reduction in insulin resistance indices, evident both after therapy (p < 0.001) and during post-therapy follow-up (p = 0.001) compared to baseline values. Furthermore, patients showed improvements in clinical hyperandrogenism and reductions in ovarian volume.
Conclusions: Our study highlights the effectiveness of low-dose metformin therapy in improving insulin resistance and metabolic parameters among adolescent patients with PCOS. Sustained benefits were observed even after treatment cessation. These findings underscore the potential for early intervention with metformin during adolescence to confer long-lasting advantages in managing metabolic abnormalities associated with PCOS.
Keywords: Adolescents; Hyperandrogenism; Insulin; Insulin resistance; Metformin; Ovary; PCOS.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Conflict of Interest: The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper. Salvatore Giovanni Vitale is an Editorial Board Member of Advances in Therapy and was not involved in the selection of peer reviewers for the manuscript nor any of the subsequent editorial decisions. Stefano Di Michele, Alice Tassi, Claudia Succu, Stefano Angioni, and Anna Maria Fulghesu have nothing to disclose. Ethical Approval: The study adhered to the Declaration of Helsinki. Informed consent was obtained from parents for each subject. Institutional review board approval was secured from the Sardinia Regional Territorial Ethics Committee (prot. no. 41, 27-5-2024 All. 2.10). Regarding informed consent, in our university gynecology outpatient clinic, it is standard practice to have patients sign a general consent form, which states that their data may be used for retrospective studies and potentially published in the future. Specifically, for adolescent and therefore underage patients, such as those included in this retrospective study, parental consent is obtained for both participation in the study and the potential publication of the data within our database.
Figures
Similar articles
-
Insulin-sensitising drugs (metformin, rosiglitazone, pioglitazone, D-chiro-inositol) for women with polycystic ovary syndrome, oligo amenorrhoea and subfertility.Cochrane Database Syst Rev. 2017 Nov 29;11(11):CD003053. doi: 10.1002/14651858.CD003053.pub6. Cochrane Database Syst Rev. 2017. PMID: 29183107 Free PMC article.
-
Comparative effects of acupuncture and metformin on insulin sensitivity in women with polycystic ovary syndrome: a systematic review and meta-analysis.Front Endocrinol (Lausanne). 2025 Jun 18;16:1553684. doi: 10.3389/fendo.2025.1553684. eCollection 2025. Front Endocrinol (Lausanne). 2025. PMID: 40607225 Free PMC article.
-
Association of insulin resistance with in vitro fertilization outcomes in women without polycystic ovarian syndrome: potential improvement with metformin treatment.Hum Reprod. 2025 Aug 1;40(8):1562-1569. doi: 10.1093/humrep/deaf100. Hum Reprod. 2025. PMID: 40441707
-
Metformin treatment before and during IVF or ICSI in women with polycystic ovary syndrome.Cochrane Database Syst Rev. 2014 Nov 18;2014(11):CD006105. doi: 10.1002/14651858.CD006105.pub3. Cochrane Database Syst Rev. 2014. Update in: Cochrane Database Syst Rev. 2020 Dec 21;12:CD006105. doi: 10.1002/14651858.CD006105.pub4. PMID: 25406011 Free PMC article. Updated.
-
Metformin and lifestyle modification in polycystic ovary syndrome: systematic review and meta-analysis.Hum Reprod Update. 2015 Sep-Oct;21(5):560-74. doi: 10.1093/humupd/dmv025. Epub 2015 Jun 9. Hum Reprod Update. 2015. PMID: 26060208
References
-
- The Rotterdam ESHRE/ASRM-Sponsored PCOS Consensus Workshop Group. Revised 2003 consensus on diagnostic criteria and long-term health risks related to polycystic ovary syndrome (PCOS). Hum Reprod. 2004;19:41–7. - PubMed
-
- Fulghesu AM, Di Michele S, Zangaris I, Cordella M, Pittui E, Scalise G, et al. Clinical and ultrasound features of normocyclic non-hyperandrogenic adolescents in early gynecological life. Gynecol Obstet Investig. 2024. 10.1159/000542393. - PubMed
-
- Kolhe JV, Chhipa AS, Butani S, Chavda V, Patel SS. PCOS and depression: common links and potential targets. Reprod Sci. 2022;29:3106–23. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical