Glycopolymer nanomicelles: pH-responsive drug delivery, endocytosis pathway, autophagy behavior, and the effect of autophagy inhibitors
- PMID: 40478478
- PMCID: PMC12144062
- DOI: 10.1007/s10856-024-06837-4
Glycopolymer nanomicelles: pH-responsive drug delivery, endocytosis pathway, autophagy behavior, and the effect of autophagy inhibitors
Abstract
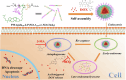
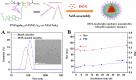
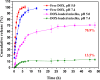
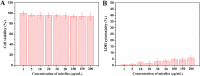
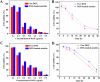
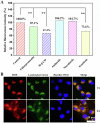
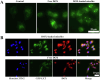
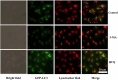
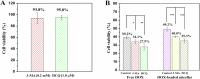
Glycopolymer drug delivery nanosystems have attracted increasing attention in the field of sustainable biomaterials and clinical biomedicine, while few studies addressed their intracellular drug delivery properties, endocytosis pathways, intracellular trafficking, autophagy behaviors and the effect of autophagy inhibitors. Based on our previous study, in this work, a pH-responsive glycopolymer (PMAgala18-b-P(MAA24-co-MAChol6)) was synthesized and used as a drug delivery carrier, to encapsulate antitumor drug doxorubicin (DOX) into nanomicelles, with high DOX loading efficiency and pH-responsive DOX release properties. The cytotoxicity, cell proliferation inhibition, endocytosis pathway, intracellular trafficking/localization, and autophagy behavior of the blank glycopolymer micelles and/or DOX-loaded micelles were studied in a Human Glioblastoma Carcinoma (H4) and green fluorescent protein-tagged H4-GFP-LC3 cell lines. The glycopolymer micelles could be taken up into the cells through favorable caveolae-mediated and clathrin-mediated endocytic pathways, and their intracellular trafficking/localization were associated with endosome-lysosome systems. Notably, after treating with DOX-loaded glycopolymer micelles (or free DOX) to the H4-GFP-LC3 cells, exogenous substances-induced autophagosome accumulation was observed. The autophagy inhibitors: 3-methyladenine (3-MA) and hydroxychloroquine (HCQ) were used to monitor the autophagy behavior of H4-GFP-LC3 cells incubated with the micelles. Interestingly, the autophagy inhibitors could significantly enhance the antitumor performance of the free DOX and/or DOX-loaded micelles, the drug combination effect of autophagy inhibitors and DOX was further studied by Bliss independent model analysis. Taken together, this work provided a preliminary understanding of the intracellular drug delivery properties of glycopolymer micelles and demonstrated the effect of different autophagy inhibitors, which might inspire future innovation of "autophagy regulator-combined nanotherapeutics" toward efficient cancer chemotherapy.
© 2024. The Author(s).
Conflict of interest statement
Compliance with ethical standards. Conflict of interest: The authors declare no competing interests.
Figures
Similar articles
-
Facile preparation of core cross-linked nanomicelles based on graft copolymers with pH responsivity and reduction sensitivity for doxorubicin delivery.Colloids Surf B Biointerfaces. 2018 Jan 1;161:606-613. doi: 10.1016/j.colsurfb.2017.11.038. Epub 2017 Nov 16. Colloids Surf B Biointerfaces. 2018. PMID: 29156337
-
Sequential release of autophagy inhibitor and chemotherapeutic drug with polymeric delivery system for oral squamous cell carcinoma therapy.Mol Pharm. 2014 May 5;11(5):1662-75. doi: 10.1021/mp5000423. Epub 2014 Apr 4. Mol Pharm. 2014. PMID: 24666011
-
Poly(ethylene glycol) shell-sheddable TAT-modified core cross-linked nano-micelles: TAT-enhanced cellular uptake and lysosomal pH-triggered doxorubicin release.Colloids Surf B Biointerfaces. 2020 Apr;188:110772. doi: 10.1016/j.colsurfb.2020.110772. Epub 2020 Jan 20. Colloids Surf B Biointerfaces. 2020. PMID: 31999965
-
Targeted delivery of doxorubicin-loaded poly (ε-caprolactone)-b-poly (N-vinylpyrrolidone) micelles enhances antitumor effect in lymphoma.PLoS One. 2014 Apr 8;9(4):e94309. doi: 10.1371/journal.pone.0094309. eCollection 2014. PLoS One. 2014. PMID: 24714166 Free PMC article.
-
Amphiphilic Diblock Terpolymer PMAgala-b-P(MAA-co-MAChol)s with Attached Galactose and Cholesterol Grafts and Their Intracellular pH-Responsive Doxorubicin Delivery.Biomacromolecules. 2016 Jan 11;17(1):98-110. doi: 10.1021/acs.biomac.5b01227. Epub 2015 Dec 28. Biomacromolecules. 2016. PMID: 26682643
References
-
- Pramudya I, Chung H. Recent progress of glycopolymer synthesis for biomedical applications. Biomater Sci. 2019;7:4848–72. 10.1039/C9BM01385G - PubMed
-
- Gerling-Driessen UIM, Hoffmann M, Schmidt S, Snyder NL, Hartmann L. Glycopolymers against pathogen infection. Chem Soc Rev. 2023;52:2617–42. 10.1039/d2cs00912a - PubMed
-
- Nagao M, Matsumoto H, Miura Y. Design of glycopolymers for controlling the interactions with lectins. Chem Asia J. 2023;18:e202300643 10.1002/asia.202300643 - PubMed
-
- Ma Z, Zhu XX. Core Cross-linked micelles made of glycopolymers bearing dopamine and cholic acid pendants. Mol Pharm. 2018;15:2348–54. 10.1021/acs.molpharmaceut.8b00205 - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

