Home-Based Transcranial Direct Current Stimulation vs Placebo for Fibromyalgia: A Randomized Clinical Trial
- PMID: 40478572
- PMCID: PMC12144624
- DOI: 10.1001/jamanetworkopen.2025.14262
Home-Based Transcranial Direct Current Stimulation vs Placebo for Fibromyalgia: A Randomized Clinical Trial
Erratum in
-
Error in the Byline.JAMA Netw Open. 2025 Jul 1;8(7):e2526572. doi: 10.1001/jamanetworkopen.2025.26572. JAMA Netw Open. 2025. PMID: 40674057 Free PMC article. No abstract available.
-
Error in the Byline.JAMA Netw Open. 2025 Oct 1;8(10):e2540518. doi: 10.1001/jamanetworkopen.2025.40518. JAMA Netw Open. 2025. PMID: 41037272 Free PMC article. No abstract available.
Abstract
Importance: Previous trials with smaller samples and shorter follow-up periods showed that multiple-session home-based anodal transcranial direct current stimulation (A-tDCS) on the left dorsolateral prefrontal cortex (DLPFC) improved fibromyalgia symptoms. However, the duration of the effect, the influence of exercise and pain neuroscience education (PNE), and the role of placebo remain unclear.
Objective: To evaluate whether A-tDCS targeting the left DLPFC, combined with exercise and PNE, is more effective than sham tDCS in reducing pain and disability, based on placebo-test responses (responders vs nonresponders).
Design, setting, and participants: This double-blind, sham-controlled randomized clinical trial enrolled women aged 18 to 65 years with fibromyalgia. Participants were randomized to receive A-tDCS or sham tDCS between April 2022 and April 2024. They were treated at home and at the outpatient Clinical Research Center of Hospital de Clínicas de Porto Alegre in Porto Alegre, Brazil. Exclusion criteria included tDCS contraindications and uncontrolled clinical conditions. Intention-to-treat analyses were conducted from July to December 2024.
Interventions: Home-based tDCS (2 mA; 20 minutes daily) or sham tDCS (2 mA; 30 seconds at the start, then 10 minutes, and then 20 minutes, with a 20-second ramp-up and ramp-down) for 4 weeks with anodal-left and cathodal-right prefrontal stimulation (35 cm2 electrodes), combined with exercise and PNE via videos and remote supervision following in-person training.
Main outcomes and measures: Change in Multidimensional Pain Interference Index (MPII) at treatment end and 3-month follow-up. MPII was measured by the Brief Pain Inventory, a 7-item scale that assesses the impact of pain on daily activities, emotional well-being, and social interactions.
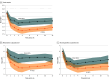
Results: A total of 112 female patients (mean [SD] age, 49.04 [9.71] years) were included and randomized to either A-tDCS (n = 56 placebo nonresponders) or sham tDCS (n = 56 placebo responders). In the intention-to-treat analysis, linear mixed-effects models showed reduced MPII by 38.76% (95% CI, -41.90% to -30.92%) for the A-tDCS group vs 16.08% (95% CI, -21.42% to -10.41%) for sham tDCS (mean difference [MD], 22.68%; 95% CI, 12.79%-40.00%; Cohen d = 0.73). A significant treatment-by-time interaction favored A-tDCS across 5 assessments, with no interaction by placebo response. In placebo responders, MPII was reduced by 34.21% (95% CI, -46.88% to -28.29%) for A-tDCS vs 18.13% (95% CI, -24.90% to 3.34%) for sham tDCS (MD, 24.23%; 95% CI, 15.80%-32.67%). Among placebo nonresponders, MPII decreases were 35.49% (95% CI, -41.21% to -29.53%) for A-tDCS vs 25.96% (95% CI, -34.31% to -20.42%) for sham tDCS (MD, 9.52%; 95% CI, 2.79%-19.78%). Improvement in MPII of 50% or more was achieved by 62.5% of participants (n = 35) in the A-tDCS group vs 37.5% (n = 21) in the sham tDCS group (relative risk, 0.60; 95% CI, 0.39-0.91).
Conclusions and relevance: This trial found that A-tDCS along with exercise and PNE improved disability due to pain, especially in placebo test responders. The findings support fibromyalgia management and enhance understanding of tDCS-related placebo effects.
Trial registration: ClinicalTrials.gov Identifier: NCT05845528.
Conflict of interest statement
Figures
References
-
- Marques AP, Santo ASDE, Berssaneti AA, Matsutani LA, Yuan SLK. Prevalence of fibromyalgia: literature review update. Rev Bras Reumatol Engl Ed. 2017;57(4):356-363. - PubMed
-
- Desai R, Jo A, Marlow NM. Risk for medication nonadherence among Medicaid enrollees with fibromyalgia: development of a validated risk prediction tool. Pain Pract. 2019;19(3):295-302. - PubMed
-
- Moore RA, Fisher E, Häuser W, et al. Pharmacological therapies for fibromyalgia (fibromyalgia syndrome) in adults - an overview of Cochrane Reviews. Cochrane Database Syst Rev. 2021;2021(8):CD013151.
-
- Perrot S, Russell IJ. More ubiquitous effects from non-pharmacologic than from pharmacologic treatments for fibromyalgia syndrome: a meta-analysis examining six core symptoms. Eur J Pain. 2014;18(8):1067-1080. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

