Current Applications of Artificial Intelligence for Fuchs Endothelial Corneal Dystrophy: A Systematic Review
- PMID: 40478592
- PMCID: PMC12155719
- DOI: 10.1167/tvst.14.6.12
Current Applications of Artificial Intelligence for Fuchs Endothelial Corneal Dystrophy: A Systematic Review
Abstract
Purpose: Fuchs endothelial corneal dystrophy (FECD) is a common, age-related cause of visual impairment. This systematic review synthesizes evidence from the literature on artificial intelligence (AI) models developed for the diagnosis and management of FECD.
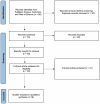
Methods: We conducted a systematic literature search in MEDLINE, PubMed, Web of Science, and Scopus from January 1, 2000, to June 31, 2024. Full-text studies utilizing AI for various clinical contexts of FECD management were included. Data extraction covered model development, predicted outcomes, validation, and model performance metrics. We graded the included studies using the Quality Assessment of Diagnostic Accuracies Studies 2 tool. This review adheres to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) recommendations.
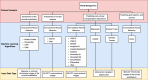
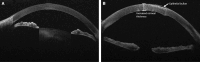
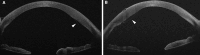
Results: Nineteen studies were analyzed. Primary AI algorithms applied in FECD diagnosis and management included neural network architectures specialized for computer vision, utilized on confocal or specular microscopy images, or anterior segment optical coherence tomography images. AI was employed in diverse clinical contexts, such as assessing corneal endothelium and edema and predicting post-corneal transplantation graft detachment and survival. Despite many studies reporting promising model performance, a notable limitation was that only three studies performed external validation. Bias introduced by patient selection processes and experimental designs was evident in the included studies.
Conclusions: Despite the potential of AI algorithms to enhance FECD diagnosis and prognostication, further work is required to evaluate their real-world applicability and clinical utility.
Translational relevance: This review offers critical insights for researchers, clinicians, and policymakers, aiding their understanding of existing AI research in FECD management and guiding future health service strategies.
Conflict of interest statement
Disclosure:
Figures


References
-
- Zoega GM, Fujisawa A, Sasaki H, et al.. Prevalence and risk factors for cornea guttata in the Reykjavik Eye Study. Ophthalmology. 2006; 113(4): 565–569. - PubMed
-
- Kitagawa K, Kojima M, Sasaki H, et al.. Prevalence of primary cornea guttata and morphology of corneal endothelium in aging Japanese and Singaporean subjects. Ophthalmic Res. 2002; 34(3): 135–138. - PubMed
-
- Higa A, Sakai H, Sawaguchi S, et al.. Prevalence of and risk factors for cornea guttata in a population-based study in a southwestern island of Japan: the Kumejima study. Arch Ophthalmol. 2011; 129(3): 332–336. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources

