Panose prevents acute-on-chronic liver failure by reducing bacterial infection in mice
- PMID: 40478727
- PMCID: PMC12259250
- DOI: 10.1172/JCI184653
Panose prevents acute-on-chronic liver failure by reducing bacterial infection in mice
Abstract
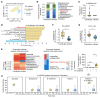
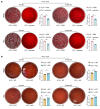
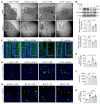
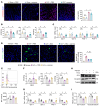
Acute-on-chronic liver failure (ACLF) is a leading cause of global liver-related mortality. Bacterial infection, especially in patients with decompensated cirrhosis, commonly triggers ACLF and is difficult to treat with antibiotics. Therefore, finding alternative strategies for preventing and managing bacterial infection is an urgent priority. Here, we observed that patients with bacterial infection and decompensated cirrhosis, as well as ACLF mice, exhibited lower fecal panose levels than uninfected controls. Megamonas funiformis, with 4α-glucanosyltransferase (4αGT) as a key enzyme for panose production, was identified as a potential panose producer. Animal experiments demonstrated that panose efficiently reduced liver injury and extended survival in ACLF mice by mitigating bacterial infection. Further results revealed that panose enhanced resistance to bacterial infection by inhibiting oxidative stress-induced gut barrier disruption, thereby limiting bacterial dissemination. Mechanistically, panose interacted with the solute carrier family 7 member 11 (SLC7A11, also known as xCT) protein to boost antioxidant glutathione levels in intestinal epithelial cells. These findings highlight panose's potential in preventing bacterial infection, offering a valuable insight into mitigating ACLF progression.
Keywords: Bacterial infections; Hepatology; Metabolism; Microbiology; Tight junctions.
Figures


Similar articles
-
Construction and validation of HBV-ACLF bacterial infection diagnosis model based on machine learning.BMC Infect Dis. 2025 Jul 1;25(1):847. doi: 10.1186/s12879-025-11199-5. BMC Infect Dis. 2025. PMID: 40596896 Free PMC article.
-
Bioinformatics analysis identifies BST1 as a potential therapeutic target linked to neutrophil extracellular traps in patients with acute liver failure.Sci Rep. 2025 Jul 8;15(1):24549. doi: 10.1038/s41598-025-05792-6. Sci Rep. 2025. PMID: 40628837 Free PMC article.
-
NIH Consensus Statement on Management of Hepatitis C: 2002.NIH Consens State Sci Statements. 2002 Jun 10-12;19(3):1-46. NIH Consens State Sci Statements. 2002. PMID: 14768714
-
Granulocyte colony-stimulating factor with or without stem or progenitor cell or growth factors infusion for people with compensated or decompensated advanced chronic liver disease.Cochrane Database Syst Rev. 2023 Jun 6;6(6):CD013532. doi: 10.1002/14651858.CD013532.pub2. Cochrane Database Syst Rev. 2023. PMID: 37278488 Free PMC article.
-
Pharmacological interventions for acute hepatitis C infection: an attempted network meta-analysis.Cochrane Database Syst Rev. 2017 Mar 13;3(3):CD011644. doi: 10.1002/14651858.CD011644.pub2. Cochrane Database Syst Rev. 2017. Update in: Cochrane Database Syst Rev. 2018 Dec 03;12:CD011644. doi: 10.1002/14651858.CD011644.pub3. PMID: 28285495 Free PMC article. Updated.
References
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

