Dynamic relocation of PKA regulatory subunits during sperm capacitation: Linking PKA to the CatSper signaling complex
- PMID: 40478882
- PMCID: PMC12167957
- DOI: 10.1073/pnas.2501741122
Dynamic relocation of PKA regulatory subunits during sperm capacitation: Linking PKA to the CatSper signaling complex
Abstract
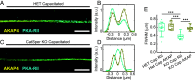
To fertilize an oocyte, mammalian spermatozoa must undergo a maturation step known as capacitation that takes place after ejaculation. Protein kinase A (PKA) plays a fundamental role in capacitation in all mammalian species. Before capacitation, PKA is maintained in an inactive state where the catalytic subunits are bound to a dimer of inhibitory regulatory subunits. A key element in the regulation of PKA lies in its intracellular compartmentalization achieved by docking at A-kinase anchoring proteins (AKAP). Despite the crucial role of the modulation of local PKA activity in fertilization, its localization and mechanism of compartmentalization are not well understood. Here, we approach this problem using quantitative laser scanning microscopy and superresolution imaging to dissect the interactions and relocalization of PKA subunits during capacitation. We find that in the resting state, both catalytic and regulatory subunits colocalize close to the axoneme. Upon capacitation, the PKA regulatory subunits, but not the catalytic subunits, relocate to a quadrilateral structure along the flagellum principal piece, in the vicinity of AKAP4 and the CatSper channel signaling complex. Furthermore, this quadrilateral localization of PKA regulatory subunits disappears in sperm from CatSper1 knockout mice. The sharp difference between the localization of PKA regulatory subunits in capacitated vs. noncapacitated sperm cells demonstrates its suitability as a biomarker for identifying capacitation, an enduring problem in the study of sperm physiology.
Keywords: Cdc42; DNA-PAINT; fertilization; protein kinase A (PKA); superresolution.
Conflict of interest statement
Competing interests statement:G.M.L., M.G.B., and Dario Krapf are shareholders of Fecundis. M.G.B., and Dario Krapf are shareholders of Fecundis, exceeding 5% each.
Figures
References
-
- Chang M. C., Fertilizing capacity of spermatozoa deposited into the fallopian tubes. Nature 168, 697–698 (1951). - PubMed
-
- Florman H. M., Fissore R. A., “Fertilization in mammals” in Knobil and Neill’s Physiology of Reproduction, Plant T. M., Zeleznik A., Eds. (Academic Press, ed. 4, 2015), pp. 149–196.
-
- Yanagimachi R., “Mammalian fertilization” in The Physiology of Reproduction, Knobil E., Neill J. D., Eds. (Raven Press, ed. 2, 1994).
MeSH terms
Substances
Grants and funding
- R01 HD106968/HD/NICHD NIH HHS/United States
- Red Federal de Microscopía de Super-resolución/Ministerio de Ciencia, Tecnología e Innovación (MINCyT)
- Keystone Grant/The Histochemical Society
- PICT 2021-1-0102/agenciaidiar | Fondo para la Investigación Científica y Tecnológica (FONCYT)
- R01HD106968/HHS | NIH (NIH)
LinkOut - more resources
Full Text Sources
Miscellaneous

