Expressing intrinsically-disordered tardigrade proteins has positive effects on acute but not chronic stress tolerance in Saccharomyces cerevisiae
- PMID: 40478923
- PMCID: PMC12143556
- DOI: 10.1371/journal.pone.0325682
Expressing intrinsically-disordered tardigrade proteins has positive effects on acute but not chronic stress tolerance in Saccharomyces cerevisiae
Abstract
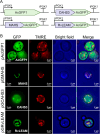
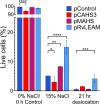
The production of high value and commodity chemicals, biopharmaceuticals and biofuels using Saccharomyces cerevisiae is hindered by various stress factors that affect yield and efficiency. Tardigrades, known for their remarkable stress tolerance, express unique proteins responsible for their resilience. This study evaluates the impact of expressing the tardigrade proteins CAHS3, MAHS, and RvLEAM on stress tolerance in S. cerevisiae. Our results show that high yields of these proteins do not impede yeast growth, except for CAHS3, which reduces proliferation. Expression of MAHS enhances acute heat tolerance, while MAHS and RvLEAM confer increased tolerance to acute hyperosmotic stress. Both CAHS3 and RvLEAM improve desiccation survival. However, these proteins do not provide benefits under chronic stress conditions such as prolonged exposure to high temperature, hyperosmotic stress, or solvents. These findings highlight the potential utility of tardigrade proteins for transient stress protection in industrial bioprocesses and suggest future engineering approaches for improved stress tolerance in yeast.
Copyright: © 2025 León López et al. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures



References
MeSH terms
LinkOut - more resources
Full Text Sources

