Recent Advances in the Use of Ionic Liquids and Deep Eutectic Solvents for Lignocellulosic Biorefineries and Biobased Chemical and Material Production
- PMID: 40479538
- PMCID: PMC12203485
- DOI: 10.1021/acs.chemrev.4c00754
Recent Advances in the Use of Ionic Liquids and Deep Eutectic Solvents for Lignocellulosic Biorefineries and Biobased Chemical and Material Production
Abstract
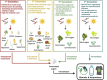
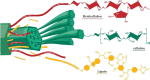
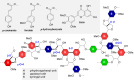
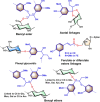
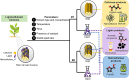
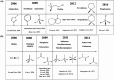
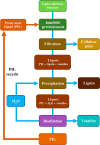
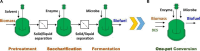
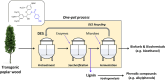
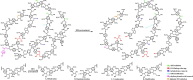
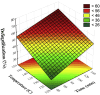
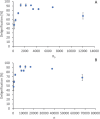
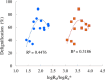
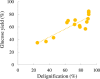
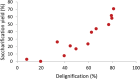
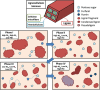
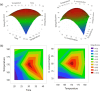
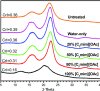
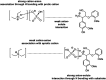
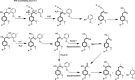
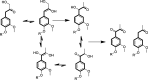
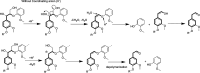
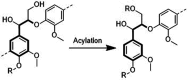
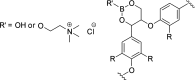
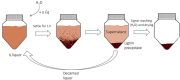
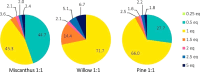
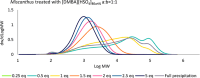
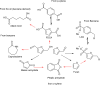
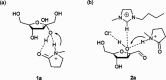
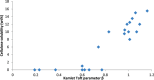
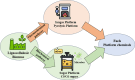
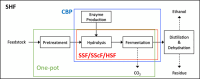
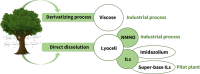
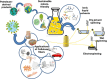
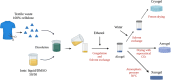
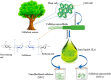
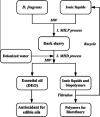
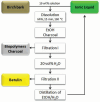
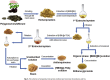
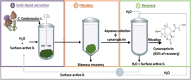
Biorefineries, which process biomass feedstocks into valuable (bio)products, aim to replace fossil fuel-based refineries to produce energy and chemicals, reducing environmental and health hazards, including climate change, and supporting a sustainable economy. In particular, lignocellulose-based biorefineries, utilizing the most abundant renewable feedstock on Earth, have significant potential to supply sustainable energy, chemicals and materials. Ionic liquids (ILs, organic salts with low melting temperatures) and deep eutectic solvents (DESs, mixtures with eutectic points lower than the ideal mixture) are capable of dissolving some of the key lignocellulose polymers, and even the whole biomass. Furthermore, they have intrinsic advantages over molecular solvents, including safer usage profiles and high tunability, which allow tailored physicochemical properties. Such properties provide unique opportunities for the development of new processes that could unlock the full potential of future biorefineries. Here, we review the current state of lignocellulosic biomass processing with ILs and DESs, with a specific focus on the pretreatment chemistry, process flow and products from each component; followed by discussions on sustainability assessments and technological challenges. We aim to inform the research community about the opportunities, challenges and perspectives in developing truly sustainable lignocellulose-based biorefineries.
Figures
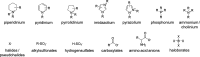








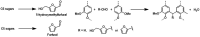




Similar articles
-
Electric fans for reducing adverse health impacts in heatwaves.Cochrane Database Syst Rev. 2012 Jul 11;2012(7):CD009888. doi: 10.1002/14651858.CD009888.pub2. Cochrane Database Syst Rev. 2012. PMID: 22786530 Free PMC article.
-
A rapid and systematic review of the clinical effectiveness and cost-effectiveness of paclitaxel, docetaxel, gemcitabine and vinorelbine in non-small-cell lung cancer.Health Technol Assess. 2001;5(32):1-195. doi: 10.3310/hta5320. Health Technol Assess. 2001. PMID: 12065068
-
Interventions to reduce harm from continued tobacco use.Cochrane Database Syst Rev. 2016 Oct 13;10(10):CD005231. doi: 10.1002/14651858.CD005231.pub3. Cochrane Database Syst Rev. 2016. PMID: 27734465 Free PMC article.
-
Behavioral interventions to reduce risk for sexual transmission of HIV among men who have sex with men.Cochrane Database Syst Rev. 2008 Jul 16;(3):CD001230. doi: 10.1002/14651858.CD001230.pub2. Cochrane Database Syst Rev. 2008. PMID: 18646068
-
Measures implemented in the school setting to contain the COVID-19 pandemic.Cochrane Database Syst Rev. 2022 Jan 17;1(1):CD015029. doi: 10.1002/14651858.CD015029. Cochrane Database Syst Rev. 2022. Update in: Cochrane Database Syst Rev. 2024 May 2;5:CD015029. doi: 10.1002/14651858.CD015029.pub2. PMID: 35037252 Free PMC article. Updated.
References
-
- Romanello M., Walawender M., Hsu S.-C., Moskeland A., Palmeiro-Silva Y., Scamman D., Ali Z., Ameli N., Angelova D., Ayeb-Karlsson S.. et al. The 2024 report of the Lancet Countdown on health and climate change: facing record-breaking threats from delayed action. Lancet. 2024;404(10465):1847–1896. doi: 10.1016/S0140-6736(24)01822-1. - DOI - PMC - PubMed
-
- Romanello M., Napoli C. d., Green C., Kennard H., Lampard P., Scamman D., Walawender M., Ali Z., Ameli N., Ayeb-Karlsson S.. et al. The 2023 report of the Lancet Countdown on health and climate change: the imperative for a health-centred response in a world facing irreversible harms. Lancet. 2023;402(10419):2346–2394. doi: 10.1016/S0140-6736(23)01859-7. - DOI - PMC - PubMed
-
- Welfle A. J., Almena A., Arshad M. N., Banks S. W., Butnar I., Chong K. J., Cooper S. G., Daly H., Garcia Freites S., Güleç F.. et al. Sustainability of bioenergy – Mapping the risks & benefits to inform future bioenergy systems. Biomass Bioenergy. 2023;177:106919. doi: 10.1016/j.biombioe.2023.106919. - DOI
-
- Adom F., Dunn J. B., Han J., Sather N.. Life-Cycle Fossil Energy Consumption and Greenhouse Gas Emissions of Bioderived Chemicals and Their Conventional Counterparts. Environ. Sci. Technol. 2014;48(24):14624–14631. doi: 10.1021/es503766e. - DOI - PubMed
- Liao Y., Koelewijn S.-F., Van den Bossche G., Van Aelst J., Van den Bosch S., Renders T., Navare K., Nicolaï T., Van Aelst K., Maesen M.. et al. A sustainable wood biorefinery for low–carbon footprint chemicals production. Science. 2020;367(6484):1385–1390. doi: 10.1126/science.aau1567. - DOI - PubMed

