Precision Treatment of Patients With GI Cancer Using Pre-emptive DPYD Genotyping/Phenotyping Plus Pharmacokinetic-Guided Dosing of 5-Fluorouracil
- PMID: 40479625
- PMCID: PMC12160085
- DOI: 10.1200/PO-25-00062
Precision Treatment of Patients With GI Cancer Using Pre-emptive DPYD Genotyping/Phenotyping Plus Pharmacokinetic-Guided Dosing of 5-Fluorouracil
Abstract
Purpose: The Clinical Pharmacogenetics Implementation Consortium (CPIC) recommends screening for four common DPYD variants to prevent severe toxicity in patients with cancer treated with fluoropyrimidines. A 50% starting dose followed by toxicity-based dose titration is advised for patients heterozygous for these variants. In this study, the appropriateness of the CPIC-recommended 5-fluorouracil (5-FU) starting dose was evaluated.
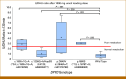
Patients and methods: Patients were grouped into four variant categories (DPYD*2A [c.1905+1G>A], DPYD*13 [c.1679T>G], c.2846A>T [p.D949V], c.1236G>A/HapB3 [p.E412E]) and a DPYD wild-type control group. Uracil loading tests were used for phenotyping. Variant patients started on a 50% reduced 5-FU dose. On the basis of steady-state 5-FU plasma concentrations, dose adjustments were made during cycles 2-4 until an 5-FU target AUC0-46h of 20-30 mg × h/L was achieved, if tolerated.
Results: Twenty-six wild-type controls and 34 DPYD variant patients were included: 16 with c.1236G>A/HapB3, eight with c.1905+1G>A, eight with p.D949V, and two with c.1679T>G. Heterozygous carriers of c.1905+1G>A (DPYD*2A) and c.1679T>G (DPYD*13) displayed significant reduced uracil metabolism. The impact on uracil clearance was highly variable in p.D949V but only minor in c.1236G>A/HapB3 variants. In all, 65% of wild-type controls had 5-FU exposure within target range on a 100% dose (mean, 23.2; IQR, 6.6). In 97% of all variant patients, the 50% reduced dose resulted in 5-FU underexposure, with a median AUC of 10.6 mg × h/L (IQR, 3.2). Dose escalation to 70% or higher was tolerated in most patients, reaching the target AUC in 68% of patients.
Conclusion: The current CPIC guidelines are overly conservative for c.1236G>A/HapB3 and most p.D949V variants. A 75% starting dose is more appropriate for most c.1236G>A/HapB3 carriers. We recommend 5-FU therapeutic drug monitoring in all patients with DPYD variants to achieve optimal 5-FU exposure.
Conflict of interest statement
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated unless otherwise noted. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to
Open Payments is a public database containing information reported by companies about payments made to US-licensed physicians (
No other potential conflicts of interest were reported.
Figures



References
-
- Henricks LM, Opdam FL, Beijnen JH, et al. : DPYD genotype-guided dose individualization to improve patient safety of fluoropyrimidine therapy: Call for a drug label update. Ann Oncol 28:2915-2922, 2017 - PubMed
-
- Van Kuilenburg ABP, Häusler P, Schalhorn A, et al. : Evaluation of 5-fluorouracil pharmacokinetics in cancer patients with a c.1905+1G>A mutation in DPYD by means of a Bayesian limited sampling strategy. Clin Pharmacokinet 51:163-174, 2012 - PubMed
-
- Fety R, Rolland F, Barberi-Heyob M, et al. : Clinical impact of pharmacokinetically-guided dose adaptation of 5- fluorouracil: Results from a multicentric randomized trial in patients with locally advanced head and neck carcinomas. Clin Cancer 4:2039-2045, 1998 - PubMed
-
- Ychou M, Duffour J, Kramar A, et al. : Individual 5-FU dose adaptation in metastatic colorectal cancer: Results of a phase II study using a bimonthly pharmacokinetically intensified LV5FU2 regimen. Cancer Chemother Pharmacol 52:282-290, 2003 - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

