Photoactivation of BODIPY Fluorescence with Green Light
- PMID: 40479737
- PMCID: PMC12318360
- DOI: 10.1021/acs.joc.5c00668
Photoactivation of BODIPY Fluorescence with Green Light
Abstract
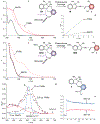
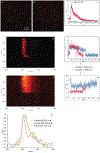
Existing synthetic dyes with photoactivatable fluorescence demand ultraviolet radiation or, at best, violet light for fluorescence photoactivation. Illumination of biological samples within this range of relatively short wavelengths, however, causes significant photodamage. Strategies for the photochemical generation of fluorescent products under irradiation at wavelengths longer than 500 nm with moderate power densities are urgently needed to enable live-cell imaging with negligible phototoxicity. We identified a possible structural design to satisfy these stringent irradiation requirements. Specifically, we demonstrated that illumination of a borondipyrromethene (BODIPY) chromophore in the green region of the visible spectrum cleaves an adjacent oxazine heterocycle to form a fluorescent product with an emission in the red spectral window. We successfully photoactivated this compound with a 561 nm laser and localized single molecules of the fluorescent product with nanometer precision under 581 nm excitation, even in the interior of live cells. Indeed, we reconstructed subdiffraction images of the nanostructured lysosomes of the labeled cells under such unprecedented illumination conditions. Our results clearly indicate that this photochemical strategy for fluorescence photoactivation is a viable one for the realization of very-much needed photoactivatable synthetic dyes for super-resolution imaging with live-cell compatible irradiation requirements.
Conflict of interest statement
The authors declare no competing financial interest
Figures



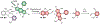


References
-
- Betzig E; Patterson GH; Sougrat R; Lindwasser OW; Olenych S; Bonifacino JS; Davidson MW; Lippincott-Schwartz J; Hess HF Imaging intracellular fluorescent proteins at nanometer resolution. Science 2006, 313, 1642–1645. - PubMed
-
- Liu Z; Lavis LD; Betzig E Imaging live-cell dynamics and structure at the single-molecule level. Mol. Cell 2015, 58, 644–659. - PubMed
-
- Sahl SJ; Hell SW; Jakobs S Fluorescence nanoscopy in cell biology. Nat. Rev. Mol. Cell Biol 2017, 18, 685–701. - PubMed
-
- Sauer M; Heilemann M Single-molecule localization microscopy in eukaryotes. Chem. Rev 2017, 117, 7478–7509. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

