Effectiveness of sacituzumab govitecan and management of neutropenia in patients with metastatic triple-negative breast cancer treated in real-world settings in the United States
- PMID: 40479864
- PMCID: PMC12173046
- DOI: 10.1016/j.esmoop.2025.105308
Effectiveness of sacituzumab govitecan and management of neutropenia in patients with metastatic triple-negative breast cancer treated in real-world settings in the United States
Abstract
Background: Sacituzumab govitecan (SG), a Trop-2-directed antibody-drug conjugate, demonstrated efficacy and manageable safety in second-line or later (2L+) metastatic triple-negative breast cancer (mTNBC) in clinical trials. We describe the real-world effectiveness of SG as 2L+ mTNBC treatment and the proportion of patients with neutropenia and its management.
Patients and methods: This study used a nationwide electronic health record-derived de-identified database. Patients with mTNBC receiving SG in 2L+ from April 2020 through June 2023 were included. Real-world overall survival (rwOS) and time to next treatment or death (TTNTD) were assessed using the Kaplan-Meier method. SG use patterns, rates of neutropenia, and granulocyte colony-stimulating factor (G-CSF) use were described.
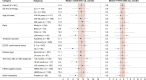
Results: For 381 patients included in the analysis, the median [interquartile range (IQR)] age was 61 years (52-69 years); 17% (n = 66) had ECOG PS ≥2; 18% (n = 70) were black; 78% (n = 298) were treated in community settings. Patients received a median (IQR) of 2 (1-3) prior treatment lines for metastatic disease. Median (IQR) SG treatment duration was 4.0 months (1.9-7.6 months) (maximum 32.7 months). At a median (IQR) follow-up of 8.7 months (4.5-14.6 months), the median rwOS and TTNTD were 11.3 months [95% confidence (CI) 10.0-12.9 months] and 5.6 months (95% CI 5.0-6.4 months), respectively. Grade 2 and 3/4 neutropenia during SG treatment occurred in 25% (n = 94) and 27% (n = 101) of patients, respectively. Any G-CSF use (primary + secondary prophylaxis and/or treatment) was observed in 59% (n = 225) of patients; 31% (n = 117) received any G-CSF prophylaxis. Any-grade neutropenia occurred in 25% (n = 29) of patients receiving any G-CSF prophylaxis versus 44% (n = 68) of patients who did not receive G-CSF.
Conclusions: SG demonstrated real-world effectiveness and manageable safety, consistent with findings from ASCENT and other published real-world studies. Patients receiving any G-CSF prophylaxis had low rates of any-grade neutropenia, suggesting SG-related neutropenia can be effectively managed with G-CSF in patients with increased risk for febrile neutropenia.
Keywords: G-CSF use; effectiveness; neutropenia; real-world clinical outcomes; sacituzumab govitecan; triple-negative breast cancer.
Copyright © 2025 The Author(s). Published by Elsevier Ltd.. All rights reserved.
Figures


References
-
- International Agency for Research on Cancer. World Health Organization Global Cancer Observatory. Breast. https://gco.iarc.who.int/media/globocan/factsheets/cancers/20-breast-fac... Available at.
-
- Breastcancer.org Breast Cancer Facts and Statistics; 2024. https://www.breastcancer.org/facts-statistics Available at.
-
- American Cancer Society Triple-negative breast cancer - details, diagnosis, and signs. 2024. https://www.cancer.org/cancer/types/breast-cancer/about/types-of-breast-...
-
- Zhang X., Yeung K.T. Metastatic triple-negative breast cancer. Curr Breast Cancer Rep. 2023;15(3):288–297.
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

