Glucocorticoid treatment in early rheumatoid arthritis is independently associated with increased PCSK9 levels: data from a randomised controlled trial
- PMID: 40480650
- PMCID: PMC12161332
- DOI: 10.1136/rmdopen-2024-005129
Glucocorticoid treatment in early rheumatoid arthritis is independently associated with increased PCSK9 levels: data from a randomised controlled trial
Abstract
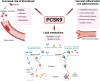
Background: Rheumatoid arthritis elevates cardiovascular disease risk. Proprotein convertase subtilisin/kexin type 9 (PCSK9), a regulator of low-density lipoprotein (LDL) metabolism, increases LDL-receptor breakdown in the liver, which elevates LDL-cholesterol levels. In addition, PCSK9 has direct effects on thrombogenesis and atherosclerotic plaque formation.We aimed to investigate (1) the impact of glucocorticoids and biological disease-modifying antirheumatic drug (bDMARD) treatments on PCSK9 and LDL-cholesterol levels, (2) whether this influence is different when autoantibodies are present and (3) the association between PCSK9 and LDL cholesterol.
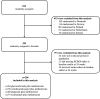
Methods: In this post hoc analysis of the NORD-STAR trial, 296 newly diagnosed patients starting methotrexate with glucocorticoids, certolizumab pegol, abatacept or tocilizumab were included. Serum PCSK9 and LDL-cholesterol levels were measured at baseline and 24 weeks. Linear regression models were used to analyse the difference in PCSK9 and LDL cholesterol between glucocorticoid and bDMARD treatments at 24 weeks. In the second analysis, the interactions between the treatment groups and autoantibody status were added to the model.
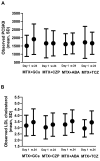
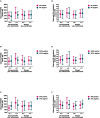
Results: After 24 weeks, PCSK9 levels were higher in the glucocorticoid group than in the combined bDMARD treatment group (-276.0 (95% CI -468.2 to -83.9)). When compared with the bDMARD treatment, these increases were more pronounced in autoantibody-positive patients. Changes in LDL cholesterol exhibited a pattern distinct from PCSK9, as it increased in all treatments.
Conclusion: Glucocorticoid treatment was associated with increased PCSK9 levels after 24 weeks. When compared with the bDMARD treatments, these increases were more pronounced in rheumatoid factor, anticitrullinated protein antibody and antinuclear antibody-positive patients. Our data provide a potential mechanistic link between glucocorticoid treatment and cardiovascular disease.
Funding: Inger Bendix Foundation for Medical Research.
Trial registration number: EudraCT2011-004720-35, NCT01491815.
Keywords: Antirheumatic Agents; Arthritis, Rheumatoid; Cardiovascular Diseases; Glucocorticoids; Lipids.
© Author(s) (or their employer(s)) 2025. Re-use permitted under CC BY. Published by BMJ Group.
Conflict of interest statement
Competing interests: KL reports institutional support for the present manuscript from Inger Bendix Foundation for Medical Research. MLH reports institutional grants from AbbVie, Bristol Myers Squibb, Eli Lilly, MSD, Pfizer, Sandoz, Novartis, Nordforsk and UCB; speaker honoraria from Pfizer, Medac, Sandoz, Novartis and UCB and institutional data safety monitoring board or advisory board fees from AbbVie. MLH has chaired the steering committee of the Danish Rheumatology Quality Registry (DANBIO, DRQ), which receives public funding from the hospital owners and funding from pharmaceutical companies. MLH cochairs EuroSpA, which generates real-world evidence of treatment of psoriatic arthritis and axial spondyloarthritis based on the secondary data and is partly funded by Novartis. TU reports speaker honoraria from Galapagos, Pfizer and UCB; participation on a data safety monitoring board or advisory board fees from UCB. DN reports research grant from MSD; consulting fees from Bristol Myers Squibb, Lilly, MSD, Novartis, Pfizer and UCB; participation on a data safety monitoring board or advisory board fees from UCB. MØ reports institutional grants from AbbVie, Amgen, Bristol Myers Squibb, Merck, Celgene, Eli Lilly, Novartis and UCB; personal speaker honoraria from AbbVie, Bristol Myers Squibb, Boehringer-Ingelheim, Celgene, Eli Lilly, Galapagos, Gilead, Hospira, Janssen, MEDAC, Merck, Novartis, Novo, Orion, Pfizer, Regeneron, Roche, Sandoz, Sanofi and UCB; participation on a data safety monitoring board or advisory board personal fees from AbbVie, Bristol Myers Squibb, Boehringer-Ingelheim, Celgene, Eli Lilly, Galapagos, Gilead, Hospira, Janssen, MEDAC, Merck, Novartis, Novo, Orion, Pfizer, Regeneron, Roche, Sandoz, Sanofi and UCB. TS-I reports grant from Amgen; speaker honoraria from Abbvie, Lipum, Novartis, Pfizer, Nordic Medicine and UCB. RFvV reports institutional grant for the present manuscript from Bristol Myers Squibb; institutional grants for research or education from Alfasigma, AstraZeneca, Bristol Myers Squibb, Galapagos, MSD, Novartis, Pfizer, Roche, Sanofi and UCB; consulting fees from AbbVie, AstraZeneca, Biogen, Bristol Myers Squibb, Galapagos, GSK, Janssen, Pfizer, RemeGen and UCB; speaker honoraria from AbbVie, AstraZeneca, Bristol Myers Squibb, Galapagos, GSK, Janssen, Pfizer and UCB; and participation on a data safety monitoring board or advisory board fees from AbbVie, AstraZeneca, Biogen, Bristol Myers Squibb, Galapagos, GSK, Janssen, Pfizer, RemeGen and UCB. All other authors declare no competing interests.
Figures
References
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous
