In-depth characterization of vaccine-induced neoantigen-specific T cells in patients with IDH1-mutant glioma undergoing personalized peptide vaccination
- PMID: 40480654
- PMCID: PMC12142095
- DOI: 10.1136/jitc-2024-011070
In-depth characterization of vaccine-induced neoantigen-specific T cells in patients with IDH1-mutant glioma undergoing personalized peptide vaccination
Abstract
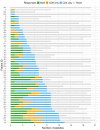
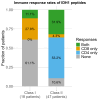
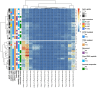
Isocitrate dehydrogenase (IDH) mutant glioma is a malignant primary brain tumor diagnosed in adults. In recent years, there has been significant progress in understanding the molecular pathogenesis and biology of these tumors. The first targeted IDH-inhibitor was approved by the US Food and Drug Administration in August 2024 for grade 2 gliomas, in light of results of a phase III trial which showed significant advantages in progression-free survival. However, biologic therapy is not curative, and subsequent treatment options offer only limited clinical benefit and often result in long-term toxicities. In addition, targeted treatment options for grade 3 and grade 4 IDH-mutant gliomas are still missing. In this study, we present n=52 patients with glioma (grade 2, 3 and 4) with confirmed IDH1 mutation (mutIDH1) in the newly diagnosed and recurrent setting who, in addition to standard-of-care, received a personalized neoantigen-targeting peptide vaccine. Each tumor was initially analyzed for somatic mutations by whole exome sequencing, and a peptide vaccine containing potential neoepitopes was designed, manufactured and vaccinated. Each vaccine consisted of peptides derived from numerous somatic mutations, including at least one peptide targeting the mutIDH1.Vaccine immunogenicity was determined by intracellular cytokine staining and simultaneous measurement of four T-cell activation markers (Interferon-γ, Tumor Necrosis Factor, Interleukin-2, CD154) after 12-day in vitro expansion of pre and post vaccination peripheral blood mononuclear cells. Extracellular CD154 staining was used to sort mutIDH1-specific CD4+T cells.Immunomonitoring revealed that the vaccines were immunogenic and induced mainly CD4 but also CD8 T cell responses. Vaccine-induced immune responses were robust and polyfunctional. Immunogenicity against mutIDH1 was high (89%). We implemented an assay which allowed us to isolate functional antigen-specific CD4+T cells in an HLA-independent manner. Subsequent T cell receptor (TCR) repertoire sequencing revealed that CD4+T cells reacting on mutIDH1 stimulation were polyclonal. Strikingly, we detected two mutIDH1-specific TCRβ candidate sequences in three different patients. These three patients had the same human leukocyte antigen (HLA) DQA-DQB alleles. The obtained TCRβ sequences could be tracked in autologous ex-vivo single-cell transcriptomic data. Our results provide a rationale for pursuing vaccination and T cell transfer strategies targeting IDH1. Furthermore, our findings indicate that personalized neoantigen-targeting vaccines might be considered for the treatment of IDH1-mutant gliomas.
Keywords: T cell; T cell Receptor - TCR; Vaccine.
© Author(s) (or their employer(s)) 2025. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ Group.
Conflict of interest statement
Competing interests: SB has ownership interests in cecava GmbH and CeGaT GmbH. DH is an employee of cecava GmbH. YB, FB, JH, MF, JJ, and JK are employed by CeGaT GmbH. OM, VS, and JW are employed by MVZ für Diagnostik, Prävention, Onkologie und Gastroenterologie Tübingen GmbH. MC has ownership interest in Cellworks Group Inc, and Personalized Cancer Medicine, PLLC. MG has consulting/advisory function for Roche, Novartis, Daiichi Sankyo, Novocure, Bayer, TME pharma, Janssen-Cilag, Seagen, cecava GmbH, CeGaT GmbH, OncoMAGNETx, and Servier. MG received honoraria from Novartis, Merck, Novocure, Medac, Kyowa, Kirin, and UCB. MG received travel support from: Medac, Novocure, Servier, and Zeiss. DR has consulting/advisory function for cecava GmbH. The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest. No competing interests were disclosed by the other authors.
Figures

References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous
